
OrthoAccel® Technologies, Inc., enrolls first patients to start 12-week orthodontic evaluation
With first patients enrolled, OrthoAccelTechnologies, Inc., announced the start of a groundbreaking clinical trial that evaluates the effect of AcceleDent on orthodontic tooth movement with aligners. Manufactured by OrthoAccel, AcceleDent is the only FDA-cleared, Class II medical device that speeds up orthodontic treatment by as much as 50 percent.
Conducted at the University of Florida College of Dentistry, the prospective randomized controlled trial follows patients for 12 weeks during their clear aligner treatment with AcceleDent. The trial’s principal investigator, Timothy Wheeler, DMD, PhD, is a distinguished researcher of orthodontic treatment with clear aligners.
“The purpose of this clinical trial is to evaluate how pulsatile forces produced through AcceleDent’s SoftPulse Technology® enable teeth to move faster,” said Wheeler, who is a professor at the University of Florida College of Dentistry. “AcceleDent is such a groundbreaking innovation that there’s a lot of interest in this study among orthodontists who want clinical evidence showing the product’s potential benefits when used with clear aligners — faster tooth movement and reduced pain.”
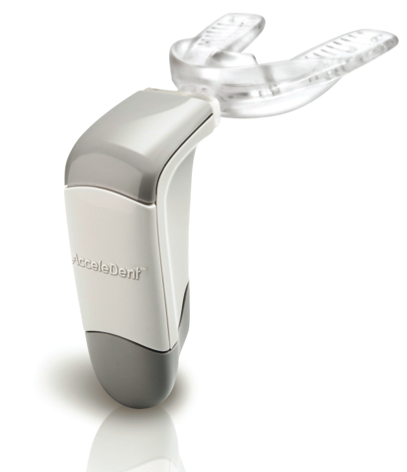
First introduced in the United States in 2012, AcceleDent’s technology was developed in part by Dr. Jeremy Mao, co-director of the Center for Craniofacial Regeneration at Columbia University College of Dental Medicine in New York. Mao’s pioneering work in the early 2000s, which was initially conducted while he was a faculty member at the University of Illinois, Chicago, was paramount in establishing the therapeutic effect of pulsatile forces in accelerating bone modeling and remodeling in the craniofacial region.
With many of the nation’s leading orthodontists prescribing AcceleDent to their patients, AcceleDent is now available in over 1,000 orthodontic locations throughout the U.S.
“As the sole provider of a noninvasive, FDA-cleared medical device that speeds up orthodontic treatment, we recognize our responsibility to support orthodontists by continuing to provide clinical evidence confirming AcceleDent’s claims of accelerating orthodontic tooth movement safely and gently,” said Michael K. Lowe, president and CEO of OrthoAccel.
Orthodontists and staff members interested in learning more about AcceleDent, or how to offer the technology at their practice, can locate an OrthoAccel sales representative at AcceleDent.com/orthodontists or call 866-866-4919.
About OrthoAccel Technologies, Inc.
Based in Houston, Texas, OrthoAccel Technologies, Inc., is a privately owned medical device company engaged in the development, manufacturing, and marketing of products to enhance dental care and orthodontic treatment. OrthoAccel developed and sells AcceleDent, the first FDA-cleared clinical approach to safely accelerate orthodontic tooth movement by applying gentle micropulses via SoftPulse Technology as a complement to existing orthodontic treatment. More information can be found at acceledent.com and acceledent.co.uk or requested via info@orthoaccel.com.
This information was provided by OrthoAccel Technologies, Inc.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores


