CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This article aims to raise awareness about the signs and treatment of obstructive sleep apnea (OSA).
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Recognize the risks of OSA in children.
- Recognize some of the symptoms of OSA in children.
- Identify some orthodontic treatment options for children with OSA.
- Realize some of the symptoms and treatments for OSA in adults.

Dr. Steven R. Olmos offers information to raise awareness about the signs and treatment of OSA
This article seeks to evaluate the 3D volumetric changes that are necessary to treat pediatric obstructive sleep apnea (OSA). Adult static therapies are not indicated for children. Children require dynamic therapies to encourage and correct skeletal development to improve sleep-breathing disorders. Formulas for arch width expansion are currently based on dental space and skeletal calculations and are not applicable nor are they validated in the treatment of pediatric OSA. Treating children with OSA requires a new formula of skeletal development for both maxilla and mandible based on correction of the immediate medical problem evaluated by overnight sleep testing called polysomnography (PSG) (attended) or home sleep testing (HST) (unattended).
The awareness and treatment for OSA is the fastest growing segment of dentistry. The Council on Dental Accreditation now requires a course in sleep pathology. The education of sleep-breathing disorders in the undergraduate dental curriculum in the United States is less than 1 hour per year.1 All of the education currently provided in dental school curriculums and most postgraduate education is based on the treatment for adults.
Successful treatment for adults includes positive pressure devices, oral appliances, oral soft tissue implants or surgery, nasal surgery, bi-maxillary advancement surgery, hypoglossal nerve stimulation, myofunctional therapy, diet, exercise, or a hybrid of any of the above. Unfortunately for most adults, OSA can only be managed for the rest of their lives.
In children, orthodontists have the ability to make significant improvement and, in some cases, cure the condition.2-3 This is significant, as children with OSA have a sevenfold risk of mortality and had greater morbidity at least 3 years before their diagnosis. After diagnosis, OSA has been associated with incidences of endocrine, nutritional, and metabolic diseases (OR 1.78, 95% CI 1.29 to 2.45), nervous conditions (OR 3.16, 95% CI 2.58 to 3.89), ENT diseases (OR 1.45, 95% CI 1.14 to 1.84), respiratory system diseases (OR 1.94, 95% CI 1.70 to 2.22), skin conditions (OR 1.42, 95% CI 1.06 to 1.89), musculoskeletal diseases (OR 1.29, 95% CI 1.01 to 1.64), congenital malformations (OR 1.83, 95% CI 1.51 to 2.22), abnormal clinical or laboratory findings.4 Children with OSA suffer from immune suppression, attention deficit hyperactivity disorders (ADHD), heart rate/blood pressure variability, neurocognitive, and endothelial inflammation.5-8
Prevalence rates for pediatric OSA range between 1.2% and 5.7%.9-11 These figures are likely low as screening for pediatric OSA is not common in most medical or dental practices.
The American Academy of Pediatrics since 2012 has made the following recommendations:
- All children/adolescents should be screened for snoring.
- Polysomnography should be performed in children/adolescents with snoring and symptoms/signs of OSAS.12
3D orthopedic treatment for pediatric OSA
Treating children with OSA requires immediate and effective therapy throughout its course to ensure proper management for this serious medical problem. Static therapies used for adults to treat OSA prevent proper skeletal development such as CPAP (headgear effect) and static oral appliances. Adult surgeries such as uvulopalatopharyngoplasty (UPPP), nasal corrective surgery, and tongue reduction are contraindicated in children.
Tonsil and adenoid surgery is effective for children for a short time; however, studies show that there is a high relapse after 6 months.13-16 Dentofacial development in snoring children is not changed by adenotonsillar surgery regardless of symptom relief as stated in otorhinolaryngology literature. It is recommended that “If snoring persists or relapses, that orthodontic maxillary widening and/or functional training should be considered. Collaboration between otorhinolaryngologist, orthodontists, and speech language pathologists is strongly recommended.”17 Enlargement of the lymphatic tissue may be a consequence of sleep-disordered breathing (SDB).18
Palatal expansion has been shown to reduce apnea, increase nasal volume, correct skeletal deformities related to breathing dysfunction, improve sleep-related symptoms such as fatigue, nocturnal enuresis, conductive hearing loss, restore proper functional nasal breathing, and uprighting head posture.19-25
Efficacy of orthodontic therapy for pediatric OSA is increased when treated at earliest onset of symptoms.26 Benefits of palatal expansion for OSA symptoms have been demonstrated to be long-standing in 12-year follow-up utilizing PSG and Epworth Sleepiness Scale (ESS).27
Palatal expansion can be accomplished with expansion devices and myofunctional exercise therapies to increase nasal volume and restore proper functional nasal breathing. A recent study quantified the three-dimensional increase of volume of the nose with palatal expansion. It was found that there is a 2.36% volume increase with each millimeter of transverse expansion (Figure 1). Another important finding is that this ratio was constant in the population base from 9- to 22-year-old patients.28 This challenges the belief that expansion is not possible in adults. Optimal outcome is accomplished with combined therapies.
Palatal expansion has been performed for many years prior to the discovery of sleep-breathing disorders. Expansion traditionally has been based on space necessary for dentition and dental alveolar bone aligned with opposing arch width, without evaluation of nasal or sleep-breathing pathology.
A historical review of expansion measurement guides has been dependent on space for teeth.
Various arch width determination methods are:
- Pont’s analysis (1909), which has been disproven long ago, determined the premolar width by multiplying the sum of the four maxillary incisors length (SI) by 100 divided by 80. The molar width is determined by SI x 100 divided by 64.
- Linder Harth Index uses the same calculations with slightly different numbers in his equation: SI x 100 divided by 85 for the premolar width and SI x 100 divided by 64 for the molar width (Figure 2).
- Korkhaus Analysis uses Linder Harth’s formula and adds the length of a line that bisects the maxillary centrals (Figure 3).
- The Bolton Analysis states that the sum of the mesiodistal widths of the 12 mandibular teeth should be 91.3% of the mesiodistal widths of the 12 maxillary teeth, and it is permissible to extract teeth to accomplish this ratio (Figure 4).
This may be the beginning of the thought process for extraction for the space provided, without regard to the underdevelopment of the arches and their relative skeletal position in regard to upper airway obstruction.
Proper functional breathing is through the nose. Air is warmed, moistened, filtered, and mixed with nitric oxide (NO) gas, which is drawn from the maxillary sinuses where it is concentrated up to 40 times. NO is important in mucociliary flow of the sinuses to ensure clearing of inhaled materials and irritants, antimicrobial effect on the lungs to prevent respiratory function, and cardiac and peripheral vasodilation that can reduce blood pressure.29-38 It has been recommended that the final endpoint in treating OSA is restoration of nasal breathing.39
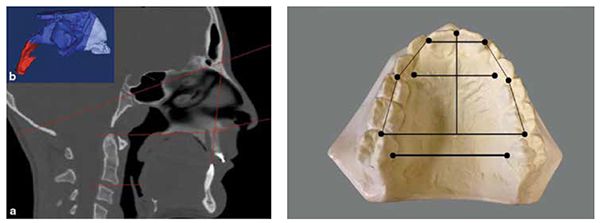
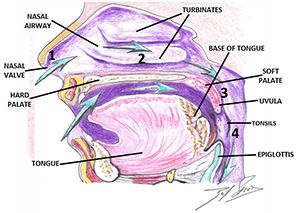
Establishing/developing patency of the four points of obstruction (Figure 5) is necessary to prevent orthodontic relapse, (anterior or posterior open bite). This is most evident in cases that have been retained with bonded anterior archwires and even orthognathic surgery with fixation plates (Figures 6-10).40 Harvold, in his work with primates, was the first to demonstrate craniofacial deformations and skeletal open bite with silicon obstruction of their noses.41
A new paradigm is proposed that the determination of expansion of the maxilla and mandible in pediatric patients with OSA be the optimal individual reduction of Apnea Hypopnea Index (AHI) and respiratory effort-related arousals (RERA), rather than the traditional space for dentition. (According to the American Academy of Sleep Medicine, AHI is an average that represents the combined number of apneas and hypopneas that occur per hour of sleep.)
Increasing oral volume and pre-venting airway collapse (vertical and phonetic bite)
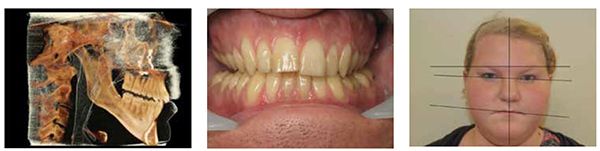
In situations where the patient has decreased lower face height and or deep overbite, they suffer from a reduced oral volume. These patients often present with canted plane of occlusion, which can predispose the patient to unilateral TM joint pathology (Figure 11). These conditions require increasing the oral volume in a three-dimensional way. Understanding that increases in volume can require small 3D changes rather than the traditional linear techniques of opening (vertical), protrusive, and lateral movements. In reality, these movements are not linear and are best described as pitch (AP cant), roll (lateral cant), and yaw (rotational cant). The coined term “Airway Centric” is a physiological 3D positioning that prevents airway muscle collapse and increases oral volume, while improving orthopedic positioning and function of the TM joints.42 This technique is known as the Sibliant Phoneme Registration, which has been shown to prevent airway collapse in adults and currently is being researched for pediatric OSA patients.43 Preventing airway collapse is key in the treatment of obstructive apnea.44
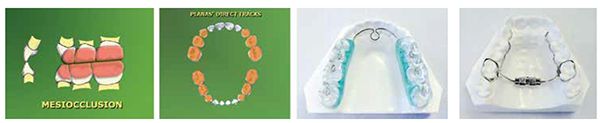
Using the Sibilant Phoneme position as a starting point for vertical stabilization corrects medio-lateral cant asymmetries, so it is an ideal technique for appliances or materials added to teeth to increase vertical (Planas Tracks or development/expansion appliances, Figures 12-14).45 The increased vertical is beneficial for inflammatory conditions of the TM joints, which is often comorbid with sleep-breathing disorders in children. Uneven loading of the TM joints in these asymmetric conditions may lead to craniofacial deformity. One in six children and adolescents have clinical signs of TMJ disorders.

Myofunctional therapy for maxillary arch development
Exercises for the tongue and skeletal muscles has been shown to be effective in the treatment of OSA.46 The tongue must have the ability for proper movement in swallowing, breathing, chewing, and speech. Evaluation for tongue tie is an important step and should be identified as early as possible. Tongue tie can result in pathology as early as breast feeding and lead to craniofacial deformities and sleep-breathing disorders as it fails to develop the palate normally.47 In a normal swallow, the dorsum of the tongue presses against the palate to develop the maxilla.
Myofunctional therapy includes exercises that are specific for developing the palate, improving lip seal, and nasal breathing.48-50 When myofunctional exercises and therapy from certified therapists are combined with oral appliance therapy for OSA, temporomandibular dysfunction (TMD), arch development, fixed vertical increase in oral volume (Planas Tracts), and orthodontic therapy, the net effect is maximized.51
Dynamic oral appliances (mandi-bular advancement) are effective treatment for pedo OSA
Static oral appliances have been shown to be effective in treating pediatric OSA; however, continued use would prevent skeletal development.52 These would include transverse expansive appliances in a linear fashion: screw, coiled NiTi springs. Examples of three dimensional expansive techniques would be NiTi wires, applied light wire force (ALF), NiTi palatal expander, quadhelix). Case study
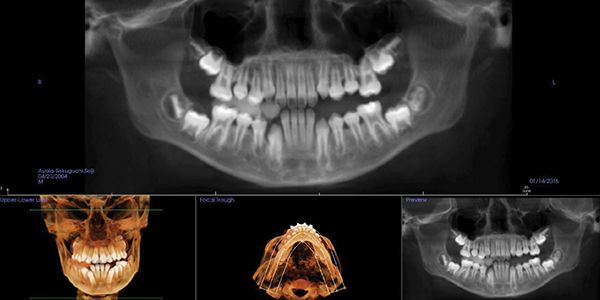
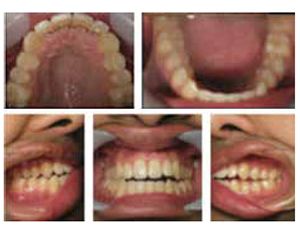
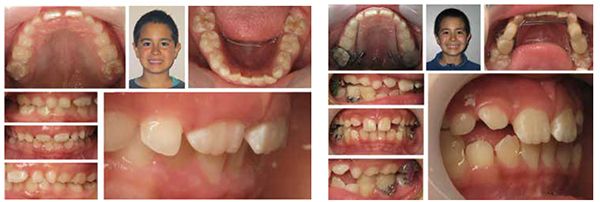
Seji, an 11-year-old boy, presented for orthodontic treatment (Figure 1).
Expansion therapy was provided (Figure 2).
By all evaluations, it would seem that the development was more than sufficient for proper arch development and dental occlusion (Figures 3-5); however, an overnight sleep study (MediByte by Braebon) read by a Board Certified Sleep Physician demonstrates that he has an AHI (apnea-hypopnea index) of 7.5. A child is diagnosed with OSA (obstructive sleep apnea) if the AHI is greater than 1. This young man has a moderate form of OSA.
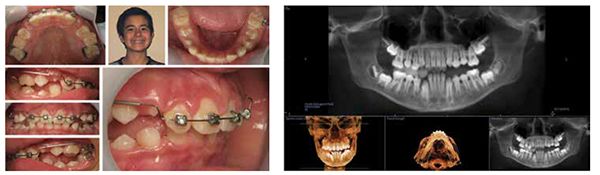

The TM joints and nasal skeletal relationships are normal after expansion (Figures 6 and 7).
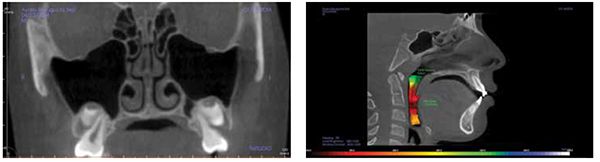
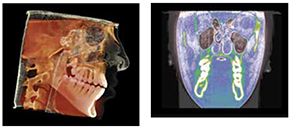
Treating pediatric OSA requires a 3D development process with re-evaluations of breathing in order to properly treat. This requires a 3D mandibular correction to open the airway and prevent collapse (Figure 8).
This is the position from which to determine orthopedic cephalometric evaluations (Figures 9 and 10).
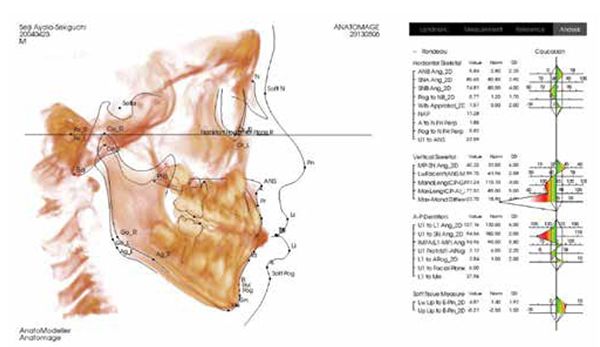
Recommend screening of all orthodontic patients for sleep-breathing disorders, including snoring utilizing the BEARS screening
Crowding of dentition is the result of retrognathia both maxilla and mandible. Maxillary retrognathia is comorbid with deviated septum and nasal obstruction. Nasal obstruction results in mouth breathing and anterior open bite (malocclusion). It is recommended that all children undergoing orthodontic therapy for underdeveloped maxilla, dental crowding, malocclusion, and open bite be evaluated for sleep-breathing disorders. The hallmark symptom is snoring as recommended by the American Academy of Pediatrics. The BEARS pediatric screening for sleep-breathing disorders is an excellent tool for this purpose. It has a range that is inclusive of toddlers, children, and adolescents.56 This tool was developed by Dr. Judith Owens, professor at Harvard and Director for Pediatric Sleep Disorders at Boston Children’s Hospital.
References
- Simmons MS, Pullinger A. Education in sleep disorders in US dental schools DDS programs. Sleep Breath. 2012;16(2):383-392.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004;27(4):761-766.
- Villa MP, Malagola C, Pagani J, Montesano M, Rizzoli A, Guilleminault C, Ronchetti R. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8(2):128-134.
- Jennum P, Ibsen R, Kjellberg J. Morbidity and mortality in children with obstructive sleep apnoea: a controlled national study. Thorax. 2013;68(10):949-954.
- Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178(3):465-474.
- Spruyt K, Gozal D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;11(4):565-577.
- Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Medicine Rev. 2014;18(2):179-189.
- Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126(5):e1161-1167.
- Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736.
- Li AM, So HK, Au CT, Ho C, Lau J, Ng SK, Abdullah VJ, Fok TF, Wing YK. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991–997.
- O’Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, Hume BC, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/ hyperactivity disorder. Pediatrics. 2003; 111(3):554–563.
- Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Ward SD, Sheldon SH, Shiffman RN, Lehmann C, Spruyt K. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-755.
- Vlahandonis A, Nixon GM, Davey MJ, Walter LM, Horne RS. A four year follow-up of sleep and respiratory measures in elementary school-aged children with sleep disordered breathing. Sleep Med. 2013;14(5):440-448.
- Biggs SN, Vlahandonis A, Anderson V, Bourke R, Nixon GM, Davey MJ, Horne RS. Long-term changes in neurocognition and behavior following treatment of sleep disordered breathing in school-aged children. Sleep. 2014;37(1):77-84.
- Bonuck KA, Chervin RD, Cole TJ, Emond A, Henderson J, Xu L, Freeman K. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34(7):875-884.
- Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129(4):e857-865.
- Löfstrand-Tideström B, Hultcrantz E. Development of craniofacial and dental arch morphology in relation to sleep disordered breathing from 4 to 12 years. Effects of adenotonsillar surgery. Int J Pediatr Otorhinolaryngol. 2010;74(2):137-143.
- Guilleminault C, Akhtar F. Pediatric sleep-disordered breathing: New evidence on its development. Sleep Med Rev. 2015;24:46-56.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004;27(4):761-766.
- Marino A, Ranieri R, Chiarotti F, Villa MP, Malagola C. Rapid maxillary expansion in children with Obstructive Sleep Apnoea Syndrome (OSAS). Eur J Paediatr Dent. 2012;13(1):57-63.
- Usumez S, Işeri H, Orhan M, Basciftci FA. Effect of rapid maxillary expansion on nocturnal enuresis. Angle Orthod. 2003;73(5):532-8.
- Schütz-Fransson U; Kurol J. Rapid maxillary expansion effects on nocturnal enuresis in children: a follow-up study. Angle Orthod. 2008;78(2):201-208.
- Eichenberger M, Baumgartner S. The impact of rapid palatal expansion on children’s general health: a literature review. Eur J Paediatr Dent. 2014;15(1):67-71.
- Cerruto C, Di Vece L, Doldo T, Giovannetti A, Polimeni A, Goracci C. A computerized photographic method to evaluate changes in head posture and scapular position following rapid palatal expansion: a pilot study. J Clin Pediatr Dent. 2012;37(2):213-218.
- McGuinness NJ, McDonald JP. Changes in natural head position observed immediately and one year after rapid maxillary expansion. Eur J Orthod. 2006;28(2):126-134.
- Villa MP, Rizzoli A, Rabasco J, Vitelli O, Pietropaoli N, Cecili M, Marino A, Malagola C. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med. 2015;16(6):709-716.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med. 2015;16(8):933–935.
- Motro M, Schauseil M, Ludwig B, Zorkun B, Mainusch S, Ateş M, Küçükkeleş N, Korbmacher-Steiner H. Rapid-maxillary expansion induced rhinological effects: a retrospective multicenter study. Eur Arch Otorhinolaryngol. 2015;Apr 3 [epub ahead of print].
- Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res. 2007;56(2):58-69.
- Mancinelli RL, McKay CP. Effects of nitric oxide and nitrogen dioxide on bacterial growth. Applied Environ Microbiol. 1983;46(1):198-202.
- Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3(1):65-70.
- Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99(12):2818-2825.
- Sanders SP, Proud D, Permutt S, Siekierski ES, Yachechko R, Liu MC. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J Allergy Clin Immunol. 2004;113(4):697-702.
- Sanders SP, Siekierski ES, Porter JD, Richards SM, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72(2):934-942.
- Jorissen M, Lefevere L, Willems T. Nasal nitric oxide. Allergy. 2001;56(11):1026-1033.
- Runer T, Cervin A, Lindberg S, Uddman R. Nitric oxide is a regulator of mucocillary activity in the upper respiratory tract. Otolaryngol Head Neck Surg. 1998;119(3):278-287.
- Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epitheial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Comm. 1993;191(1):83-88.
- Lindberg S, Cervin A, Runer T. Low levels of nasal nitric oxide (NO) correlate to impaired mucocillary function in the upper airways. Acta Otolaryngol. 1997;117(5)728-734.
- Guilleminault C, Sullivan SS. Towards Restoration of Continuous Nasal Breathing as The Ultimate Treatment Goal in Pediatric Obstructive Sleep Apnea. Pediatr Neonatol Biol. 2014;1(1). https://enlivenarchive.org/pediatrics-neonatal-biology-001.pdf. Accessed February 19, 2016.
- Olmos S. CBCT in the evaluation of airway-minimizing orthodontic relapse. Orthodontic Practice US. 2015;6(2):46-49.
- Harvold EP, Tomer BS, Vargervik K, Chierici G. Primate experiments on oral respiration. Am J Orthod. 1981;79(4):359-372.
- Moeller JL, Paskay LC. Gelb M. Myofunctional Therapy: A Novel Treatment of Pediatric Sleep-Disordered Breathing. Sleep Medicine Clinics. 2014;9(2). https://www.sleep.theclinics.com/article/S1556-407X%2814%2900025-3/references. Accessed February 19, 2016.
- Singh GD, Olmos S. Use of the sibilant phoneme registration protocol to prevent upper airway collapse in patients with TMD. Sleep Breath. 2007;11(4):209-216.
- Ng AT, Qian J, Cistulli PA. Orophayrngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29(5):666-671.
- Rivera-Morales WC, Mohl ND. Anteroposterior and mediolateral variability of the closest speaking space. Int J Prosthodont. 1990;3(2):179-184.
- Guimarães KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179(10):962-966.
- Olivi G, Signore A, Olivi M, Genovese MD. Lingual frenectomy: Functional evaluation and new therapeutical approach. Eur J Paediatr Dent. 2012;13(2):101-106.
- Rogers AP. Exercises for the development of muscles of face with view to increasing their functional activity. Dental Cosmos LX. 1918;59:857-876.
- Guimaraes KC. [Soft tissue changes of the oropharynx in patients with obstructive sleep apnea]. J Bras Fonoaudiol. 1999;1(1):69-75.
- Guilleminault C, Huang YS, Monteyrol PJ, Sato R, Quo S, Lin CH. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013;14(6):518-525.
- Cunali PA, Almeida FR, Santos CD, Valdrichi NY, Nascimento LS, Dal-Fabbro C, Tufik S, Bittencourt LR. Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath. 2011;15(4):717-727.
- Nazarali N, Altalibi M, Nazarali S, Major MP, Flores-Mir C, Major PW. Mandibular advancement appliances for the treatment of paediatric obstructive sleep apnea: a systematic review. Eur J Orthod. 2015;37(6):618-626.
- Gerbino G, Bianchi FA, Verzé L, Ramieri G. Soft tissue changes after maxillo-mandibular advancement in OSAS patients: A three-dimensional study. J Craniomaxillofac Surg. 2014;42(1):66–72.
- Lal C, White DR, Joseph JE, van Bakergem K, LaRosa A. Sleep-disordered breathing in down syndrome. Chest. 2015;147(2):570-579.
- Peanchitlertkajorn S. RPE and Orthodontic Protraction Facemask As An Alternative Therapy for Severe Obstructive Sleep Apnea Associated with Maxillary Hypoplasia. Journal of Dental Sleep Medicine. 2016;3(1)1. https://www.jdsm.org/ViewArticle.aspx?pid=30401. Accessed February 19, 2016.
- Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6(1):63-69.