CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This article aims to discuss the all too familiar esthetically negative side effects of
demineralized white spot lesions (WSL).
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions on page 43 to
earn 2 hours of CE from reading this article. Correctly answering the questions will
demonstrate the reader can:
- Read some history on dental caries.
- Identify some ways to keep tooth surfaces free of plaque.
- Identify some ways to introduce fluoride into patients’ treatment.

Drs. George J. Cisneros, Matthew Miller, and Shira Bernstein, BA, research a longstanding issue
for orthodontists
Historical perspective
While orthodontics can markedly improve our patients’ sense of well-being, as they leave our offices with corrected mal-occlusions, properly aligned teeth, and a significant boost in self-confidence, such positive gains can become shattered by the all too familiar esthetically negative side effect of demineralized white spot lesions (WSL). Such frustrating consequences can certainly diminish a clinician’s practice reputation and in the extreme may even lead to litigious follow-up. The fact that our care is focused on the 11- to 17-year-old patient population, a group very susceptible to WSLs, should keep us on the lookout for potential problems. But this has been an issue that humanity has been battling with throughout the millennia!
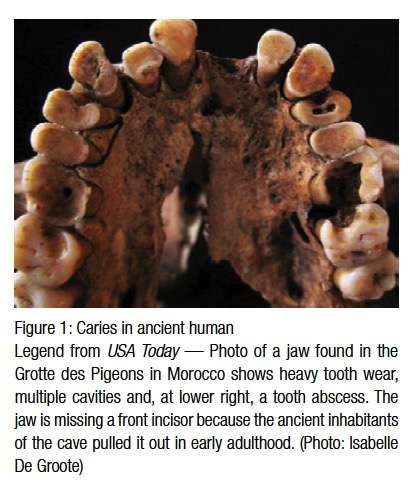 Since the advent of civilization, dental caries have been the scourge of humankind. There is evidence that hominids such as Australopithecus suffered from cavities.1 Archeology has proven that there was a sharp spike in dental caries during the Neolithic period — roughly 10,200 bc to 2000 bc. Archeologists believe the Neolithic Revolution, with its domestication of horticulture, contributed to an increase of ingested plants and carbohydrates that caused this spike2 (Figure 1). There was a belief among ancient civilizations that dental caries were caused by a dental worm, and the ADA website has a copy of an ancient Sumerian manuscript that subscribes to this belief.3 The next uptick in dental caries came during the Middle Ages due to availability of sugar cane to the Western world.4 With the 17th century came the Enlightenment, which questioned many old beliefs, including the dental worm theory.5 The first person to reject the theory of dental worms was Pierre Fauchard. He theorized that sugar was responsible for dental caries, and for this he is known as “the father of modern dentistry.”6 The 1850s saw another sharp increase in the prevalence of dental caries. Dental historians attributed this to an overall change in worldwide diet as a result of the Industrial Revolution, which brought with it a wide availability of processed foods like white sugar, refined flour, bread, and sweetened tea.7 Before the Industrial Revolution, the most common type of caries was cervical or root caries, but with the advent of processed foods, pit and fissure caries became the most common form of caries. In the 1890s, Dr. W.D. Miller hypothesized that there were bacteria in the oral cavity, and when fermentable carbo-hydrates were ingested, an acidic byproduct was produced causing caries formation.8 Dr. Miller’s hypothesis later became known as the “chemoparasitic caries theory.”9 Drs. G.V. Black and J.L. Williams’ research on dental plaque also contributed to the modern explanation of how dental caries form. In 1924, Killian Clarke first suggested Streptococcus mutans as the primary organism responsible for dental caries.10 Today we know that Streptococcus mutans and Lactobacilli are the primary bacterial sources for dental caries.
Since the advent of civilization, dental caries have been the scourge of humankind. There is evidence that hominids such as Australopithecus suffered from cavities.1 Archeology has proven that there was a sharp spike in dental caries during the Neolithic period — roughly 10,200 bc to 2000 bc. Archeologists believe the Neolithic Revolution, with its domestication of horticulture, contributed to an increase of ingested plants and carbohydrates that caused this spike2 (Figure 1). There was a belief among ancient civilizations that dental caries were caused by a dental worm, and the ADA website has a copy of an ancient Sumerian manuscript that subscribes to this belief.3 The next uptick in dental caries came during the Middle Ages due to availability of sugar cane to the Western world.4 With the 17th century came the Enlightenment, which questioned many old beliefs, including the dental worm theory.5 The first person to reject the theory of dental worms was Pierre Fauchard. He theorized that sugar was responsible for dental caries, and for this he is known as “the father of modern dentistry.”6 The 1850s saw another sharp increase in the prevalence of dental caries. Dental historians attributed this to an overall change in worldwide diet as a result of the Industrial Revolution, which brought with it a wide availability of processed foods like white sugar, refined flour, bread, and sweetened tea.7 Before the Industrial Revolution, the most common type of caries was cervical or root caries, but with the advent of processed foods, pit and fissure caries became the most common form of caries. In the 1890s, Dr. W.D. Miller hypothesized that there were bacteria in the oral cavity, and when fermentable carbo-hydrates were ingested, an acidic byproduct was produced causing caries formation.8 Dr. Miller’s hypothesis later became known as the “chemoparasitic caries theory.”9 Drs. G.V. Black and J.L. Williams’ research on dental plaque also contributed to the modern explanation of how dental caries form. In 1924, Killian Clarke first suggested Streptococcus mutans as the primary organism responsible for dental caries.10 Today we know that Streptococcus mutans and Lactobacilli are the primary bacterial sources for dental caries.
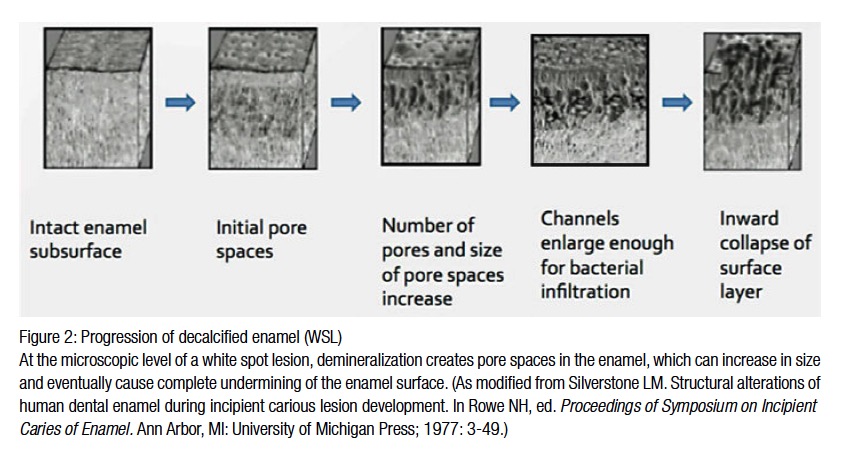
When Streptococcus mutans and Lactobacilli are combined with fermentable carbohydrates, such as glucose, fructose, or sucrose, lactic acid is produced resulting in a decrease in the intraoral pH, creating an acidic environment initiating enamel decalcification. Intraoral pH rebounds after 30-60 minutes due to the body’s natural buffering capacity, but by then enough time has occurred for the decay process to begin. Dr. Robert Stephan was first to describe this pH change in 1943. Once lactic acid is introduced into the oral environment, there is a rapid drop in pH that rebounds at a much slower rate than it took for it to drop. There must be three factors present: the surface of the tooth, the bacteria, and fermentable carbohydrates. As the intraoral pH drops below 5.5, enamel begins to demineralize, the initial step in dental caries formation (Figures 2 and 3).

Caries do not form immediately on healthy teeth, as our teeth naturally develop a biofilm that forms from proteins from our food and bacteria that colonize on the tooth surface.11 Interestingly, our teeth are the only natural surface of our body that does not shed, facilitating bacteria colonization on our teeth allowing dental caries to eventually develop.12
Over the centuries, humans have grappled with the problem of having to keep the surfaces of their teeth free of plaque. No matter the method or device, the goal has always been to remove this soft plaque and ensure that decay does not occur. Brushing twice every day with fluoride toothpaste, using mouthwash, and flossing daily has been part of the therapeutic dental mantra for decades. The effectiveness of this method has been well documented, supporting neglect as the primary cause for caries development. As there is a need for proper oral hygiene instruction, our patients are not the only negligent parties, simply because such routine instruction rarely occurs in today’s orthodontic offices.
Fluoride has evolved as our main preventative chemotherapeutic agent against caries. As stated previously, once an acidic environment develops in the oral cavity, enamel begins to demineralize resulting in white spot lesions (WSL) and eventually decay. If fluoride is introduced early enough, a chemical reaction occurs, whereby the hydroxyapatite crystals in the enamel lose their OH-ion and are replaced with an F-ion, leading to the formation of fluorapatite. This process has proven to occur even when mere trace amounts of fluoride are present in the mouth, with 0.01-10 parts per million (ppm) being sufficient.13 Aside from being added to most toothpastes sold in the United States, fluoride has been added to mouthwashes and restorative materials such as resin-modified glass ionomer (RMGI).
Another common method to introduce fluoride to the public is by adding it to the tap water. Although controversial in some areas, fluoridated water is delivered to approximately two-thirds of the U.S. population.14 It has been estimated that children who brush with fluoridated toothpaste and are exposed to fluoridated water have an 18%-40% reduction in caries.15
Another method used to introduce fluoride into patients’ mouths is through fluoride varnishes.16 Varnishes are available for professional use in a dental office, as well as for home use. The office products have 5% NaF varnish with a concentration of 22,600 ppm of fluoride. They are painted onto teeth, but care should be given to ensure that the teeth are absolutely dry before the varnish is applied, as it will adhere to only a dry enamel surface. The patient is then instructed to not eat or drink for 30 minutes to allow the varnish to sufficiently penetrate the tooth structure. The at-home varnish contains 100-1,500 ppm with the same instructions. Office fluoride gel treatments are also available for patients with weak enamel. A tray of 1.23% fluoride gel with a concentration of 12,300 ppm of fluoride is applied directly to the dentition and let sit for 4 minutes, delivering a high dose of fluoride.17
MI Paste Plus™ is another product on the market, manufactured by GC America. MI Paste contains Recaldent™, which is made by Recaldent Pty Ltd., and marketed as an anti-sensitivity agent. Dental sensitivity is caused by the wearing away of the enamel surface. This can be due to mechanical forces such as bruxism, or chemical forces such as patients suffering from gastroesophageal reflux disease (GERD). Its active ingredients are casein phosphopeptide (CPP) and amorphous calcium phosphate (ACP). Such agents work with fluoride to deliver calcium and phosphate ions to the enamel and into the oral environment. This allows the enamel surface to remineralize, preventing sensitivity from occurring.18,19,20,21
But what is it that we can do in our daily practices to create a more proactive approach in dealing with a problem that continues to be all too prevalent in contemporary society? Is there something that we can do as oral health providers to protect our patients that can fit readily within our practice regimens so that we can continue to create not only functional and beautiful smiles, but ones that are healthy as well?
References
- University of Illinois at Chicago. Epidemiology of Dental Disease online course notes. https://www.uic.edu/classes/osci/osci590/11_1Epidemiology.htm.
- Richards MP. A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence. Eur J Clin Nutr. 2002;56(12):16.
- American Dental Association. History of Dentistry Timeline. https://www.ada.org/en/about-the-ada/ada-historyand-presidents-of-the-ada/ada-history-of-dentistry-timeline.
- University of Illinois at Chicago. Epidemiology of Dental Disease online course noes. https://www.uic.edu/classes/osci/osci590/11_1Epidemiology.htm.
- Gerabek WE. The tooth-worm: historical aspects of a popular medical belief. Clinical Oral Investig. 1999;3(1):1–6.
- de Vaux JC. Who is Pierre Fauchard. Pierre Fauchard Academy Web site. https://www.fauchard.org/publications/47-who-is-pierre-fauchard.
- Suddick RP, Harris NO. Historical perspectives of oral biology: a series. Crit Rev Oral Biol Med. 1990;1(2):135-51.
- Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med. 2002;13(2):108-125.
- Baehni PC, Guggenheim B. Potential of diagnostic microbiology for treatment and prognosis of dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7(3):259–277.
- Grönroos L. Quantitative and Qualitative Characterization of Mutans Streptococci in Saliva and in Dentition. [dissertation]. Helsinki: University of Helsinki; 2000.
- Penn State University. BioFilm Primer online. https://www. personal.psu.edu/faculty/j/e/jel5/biofilms/primer.html
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(2):279–294.
- Rošin-Grget K, Peroš K, Sutej I, Bašić K. The cariostatic mechanisms of fluoride. Acta Med Acad.
2013;42(2):179-188. - American Dental Association. Fluoridation Facts. https://www.ada.org/~/media/ADA/Member%20Center/FIles/fluoridation_ facts.ashx .
- CDC. Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR Recomm Rep. 2001;50(RR-14):1–42. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5014a1.htm
- DA Division of Communications, Journal of the American Dental Association, ADA Council on Scientific Affairs. Fluoride treatments in the dental office extra protection for your
teeth. J Am Dent Assoc. 2007;138(3):420. https://www.ada.org/~/media/ADA/Member%20Center/FIles/patient_72.
ashx - Newbrun E. Topical fluorides in caries prevention and management: a North American perspective. J Dent Educ. 2001;65(10):1078-1083.
- Reynolds EC. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: a review. Spec Care Dentist. 1998;18(1):8-16.
- Reynolds EC. The role of phosphopeptides in caries prevention. Dent Perspectives. 1999;3:6-7.
- Sato T, Yamanaka K, Yoshii E. Caries prevention potential of a tooth-coating material containing casein phosphopeptide – amorphous calcium phosphate (CPP-ACP). [abstract].
International Association for Dental Research general session: Goteborg; 2003. - Reynolds EC, Cain CJ. Webber FL, Black CL, Riley PF, Johnson IH, Perich JW. Anticariogenicity of tryptic caseinand synthetic-phosphopeptides in the rat. J Dent Res.
OP 1995;74:1272-1279.






