Dr. John L. Hayes looks at the influence of the Carolina Research papers on early Class II treatment.
 Dr. John L. Hayes discusses the influence of the Carolina Research papers on one- or two-phase orthodontic treatment
Dr. John L. Hayes discusses the influence of the Carolina Research papers on one- or two-phase orthodontic treatment
The purpose of this article is to evaluate the validity of the Carolina Research papers of 1997, 1998, and 2004.1,2,3 The Carolina Research was designed to answer the following questions: Should the approach for CL II care be “early treatment /phase 1 care — followed by a phase 2” or should it be “later care – 1 phase of care”?
In the end, the major conclusion of the Carolina Research follows:
We conclude that, for children with moderate to severe CL II problems, early treatment [a phase 1 of care] followed by later comprehensive treatment [a second phase of care] on average does not produce major differences in jaw relationship or dental occlusion [than waiting to start later—with 1 phase of care].2
Given some passage of time, the research conclusion came to be accepted as “all early treatment is a waste of time and money,” and some folks were stirred up enough to call for those who practiced “early treatment” to be pilloried.
The Carolina Research was very persuasive — enough to cause easy acceptance and marching orders. Why was it persuasive? Several reasons may be responsible for the “willing suspension of disbelief”:
- Esteemed institutions were involved.
- Esteemed prime movers were involved.
- The research was touted as a true RCT (randomized clinical trial) with the reliability of a true RCT — “…the first ever RCT for orthodontic CL II research.”4
- The research passed all the stringent peer-review hurdles.
It should be fair to say that these papers did more to influence orthodontic diagnosis, treatment planning, and delivery of orthodontic care than any such research before or since — and not always in a good way, in the opinion of many. For the last quarter-century, the influence of these papers has been immeasurable — reaching beyond orthodontics.

When a true RCT has been accomplished — with a successful intervention — it is the most robust evidence for “science”; we are generally speaking of a medical RCT. 17 Some orthodontic research, although not meeting the requirements of a true RCT, has been referred to as “RCT research.” Scientists know that just because something is called an RCT does not make it so.
The conclusion of the referenced research was suspect from the outset because the research design and implementation were not consistent with a true RCT.5 The 1998 research methodology and conclusion, if placed on a validity scale of “science” at one end and “opinion” at the other end, would reside closer to the opinion end.
An early misstep with the Carolina Research1,2,3 is evident from the basic research design: A CL II malocclusion is not a diagnosis, but rather — it is a symptom of some diagnosis. The research purpose apparently was to evaluate CL II symptoms inappropriately using a RCT as a kind of umbrella. RCTs are used to evaluate efficacy of an etiology. And symptoms are not etiologies. It is reasonable to consider a CL II, at least partially, as a symptom of “maxillary transverse deficiency” (a strong etiology theory… holding for decades now).6-14 The Carolina Research did not mention any possible CL II etiology.
In cases where the maxilla is too narrow in relationship to the mandibular skeletal transverse width, the mandible is caused to fit in a CL II retro position during growth. It is well-known that phase 1 care can improve the transverse by way of (nonsurgical) skeletal RPE expansion to create room enough for the mandible to grow anteriorly — given normal growth potential and early enough start (usually with help from an indexed Hawley of some type).13,14
The Carolina Research did not investigate, measure, or treat the deficient maxillary skeletal transverse dimension present with its CL II patients. The transverse dimension differential (maxillary transverse dimension as it relates to the mandibular transverse) is a “confounding variable,” and in this instance, it is also a “prognostic factor.”6,8,9,10,11 The Carolina Research did not address those biases, which in turn contributed to a non-evidential report. (Figures 2, 3, 4) Unfortunately, the Carolina study treated CL II symptoms only.
A RCT requires that we identify the etiology — treat the etiology — test the intervention (along with an untested control group), and if the intervention is effective — success!
The four definitions that follow are the helpful to understand the backbones of a true RCT: No. 1 The Scientific Method; No. 2 “Confounding” variable (the non-control of which would be a serious bias); No. 3 “Prognostic” factor or variable (the non-control of which would be a serious bias), and No. 4 “A Randomized Clinical Trial,” RCT.
No. 1 The Scientific Method
The regimen — 1. Formulate a hypothesis. 2. Gather data. 3. Analyze the data. 4. Then determine whether the findings support the hypothesis or not. Accept the hypothesis, or adjust the hypothesis, and repeat the process as necessary. “Nothing gets discovered without the Scientific Method” according to Sir Francis Bacon, (1561-1626). Therefore, Bacon would likely say that the answer to questions regarding early versus late CL II care would require the application of the scientific method.
No. 2 Confounding variables
The non-identification and non-control of confounding variables would be a serious bias. “Confounding bias is often referred to as a ‘mixing of effects’ wherein the effects of the exposure under study on a given outcome are mixed in with the effects of an additional factor (or set of factors) resulting in a distortion of the true relationship. In a clinical trial, this can happen when the distribution of a known prognostic factor differs between groups being compared.”15
For example, there are six varieties of CL II malocclusions, with possibly six different regimens to best treat each diagnosis; given that the patients were then treated in the Carolina study without regard to their specific CL II diagnosis, it would ensure some were treated less appropriately than others — introducing unwanted biases.18
Another example would be when the male and female data were homogenized as in the Carolina Research, the effects of earlier female skeletal maturity along with later male skeletal maturity caused age-sensitive data to be muddled — introducing unwanted biases.
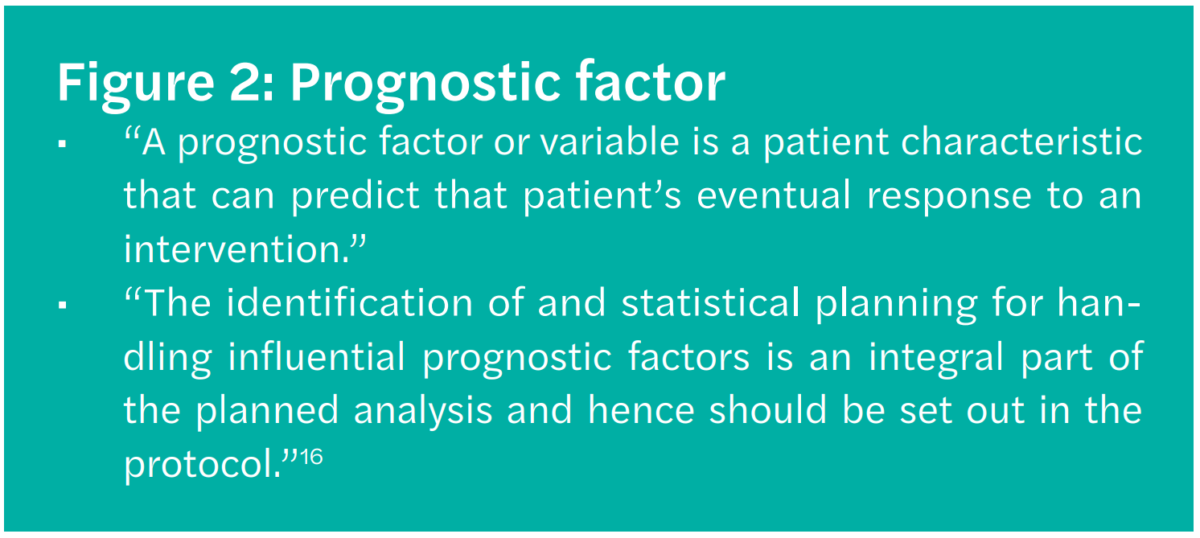
No. 3 Prognostic factor (Figure 2)
Non-identification and non-control of any prognostic factor would create a serious bias. “The identification of and statistical planning for handling influential prognostic factors is an integral part of the planned analysis and hence should be set out in the protocol.”16
For example, if CL II situations were to be heavily influenced by the transverse skeletal harmony present with the maxilla as related to the mandible, a transverse deficiency would be at least partially prognostic (the etiology). Accordingly, the measurement of each patient’s skeletal transverse situation would be important. Some CL II patients are more transverse deficient than others; mildly deficient patient would be expected to respond to a reasonable regimen better that a severely deficient patient. The Carolina study had no idea which patients were which in the transverse skeletal arena (no handle on the prognostic factor), and thus, the data was muddled with biased results.

No. 4 Randomized clinical trial (Figures 3 and 4)
“(RCTs] remain the most robust research method available to find the real effect of an intervention, but a biased RCT [our emphasis] can lead to the adoption of a wasteful intervention and may even harm patients.”17
“In all likelihood, no matter how many variables one adjusts for, there will be residual confounding, possibly by factors that are unknown and cannot be measured.”16

Discussion of the Carolina Research
We do not believe that anything was done intentionally to present false information by the authors; however, the 1998 Carolina Research did not meet basic minimum standards to be called a true RCT — which it was touted to do on more than one occasion.4 The conclusion did not reach the level of “evidential” or “science” as promoted. The conclusion has been considered harmful to patients by some. Of the 12 RCT characteristics evaluated, the research only met one (prospective design) (Figure 4).
The first part of the two-part Carolina Research was the application of phase 1 care to one group while holding in abeyance a “symptom matching group” to be treated later in one phase for eventual comparison.2 It has been argued that the phase 1 methodology employed was a setup to place the worst foot forward and did not evaluate some of the most promising CL II regimens (such as maxillary expansion). The Carolina Research did evaluate both HG and Bionator; however, it should be reasonable to assume that the effectiveness of those regimens was effectively crippled because of the lack of adjunctive help from maxillary expansion.
If the mandible is desired to grow into its CL I potential, the maxillary skeletal width needs to be wide enough to accommodate the mandible,11 and the patient needs to be young enough (notably for females).
The 1998 study conclusion has been seen by some as a detriment to young patients who have missed out on phase 1 care and missed out on lifelong advantages. The study has also been seen by some as a detriment to our colleagues — the oral surgeons whose patients may have experienced unexpected surgical relapses potentially caused by dental expansion. Dental transverse expansion is more common with later care. After orthognathic surgery, dental expansion tends to relapse and might be contributory to later surgical relapse.
There is no doubt that CL II correction is important. A fair question: How successful is late treatment CL II care? Successful long-term late treatment results are probably out there. One so-called success report revealed several case failures with excessive relapse.19 The failures and partial failures are likely related to failure to address the etiology. Accordingly, 1998 Carolina Research patients (either early or late treated) would have revealed even more CL II failures — had they been followed.
If the cause (or part of the cause) of a CL II malocclusion could be addressed early on, the results in the long term would be more stable.
Comparison of early with late treatment effectiveness
- Anterior open bite (AOB). Late treatment with surgery or ortho, in addition to surgery, has not proven as effective as hoped. We could not find one case that was considered a success after looking over 50 AOB studies each with several subjects. In our experience, early treatment addressing etiology has opportunity for success.7
- Impacted canines. Late treatment increases the likelihood for surgery and/or loss of teeth. Proper phase I care can help improve or avoid impactions.12
- Poor facial growth. How effective is late treatment for poor facial growth? Late treatment is more frequently linked with orthognathic surgery. A transverse deficient maxilla is a prognostic factor for a CL II malocclusion and other skeletal problems. A transverse deficient maxilla also causes a transverse deficient nasal airway with related medical maladies.
- Airway-related medical maladies. Early, nonsurgical RPE to help address maxillary skeletal transverse deficiency works best early on, as soon as the malady is diag This type of deformity may be first noticed by an orthodontist.
- CL II correction. This pattern of poor growth can be improved by starting early enough to reduce distal pressure on the condyles by gaining accommodating skeletal maxillary width while encouraging the mandible into CL I with a Hawley bite plate, as one example of many.20 Patients are being successfully treated without surgery and with improved stability — a win-win.
- Science. The recommendation of the Carolina study was that early intervention care was essentially wasteful in time and money compared to waiting for later treatment. That turned out to be closer to an opinion than science. The 1998 study on the benefit of early CL II treatment was not science because it was not a true RCT.
- When to start. A parent’s preference (with either early intervention or later orthodontic care) plays the strongest role in starting. Trust in the family dentist and advice from an orthodontist can help tip the scale in one direction or another. It should be clear that both “later treatment” and “early intervention” are not yet supported by “science” (by way of a true RCT) — and that is OK given the multiple research constraints of such proof. In our opinion, a particular early treatment regimen has an edge in being eventually backed as “science.”6-12 A prognostic factor (etiology) for some malocclusions has been identified for decades now and has passed a pilot study followed by several thousand successful cases in one office. There are other reports; thus, a modified RCT could be planned. Numerous airway studies illustrate the important role an orthodontist can play early on with some airway-related medical maladies such as ADD, ADHD, and obstructive sleep apnea (OSA) among others.
After reading Dr. Hayes’ article on early Class II treatment, read Dr. Todd Bovenizer’s article where he evaluates this Class II treatment with the end result in mind. https://orthopracticeus.com/treatment-of-asymmetrical-class-ii-malocclusion/
- Tulloch, et al. Benefit of early Class II treatment: progress report of a two-phase randomized clinical trial. Am J Orthod Dentofacial Orthop. 1998;113(1):62-72.
- Tulloch JF, Proffit WR, Phillips C. Influences on the outcome of early treatment for Class II malocclusions. Am J Orthod Dentofacial Orthop. 1997; 111(5):533-542.
- Tulloch JF, Proffit WR, Phillips C. Outcomes in a 2-phase randomized clinical trial of early Class II treatment. Am J Orthod Dentofacial Orthop. 2004;125(6):657-667.
- Proffit WR. Seminar. The world’s first gold standard orthodontic RCT research ever published: a randomized clinical trial. PAO Meeting (The Greenbrier) 1987. Presented at 50th Annual Penn Orthodontic Alumni Meeting (Philadelphia); 2003.
- Hamilton DC. Critique of: Tulloch, et.al. Benefit of early Class II treatment: progress report or a two-phase randomized clinical trial. 1998. Presented at AAO National Meeting, Toronto, 2001; Tape 32B.
- Hayes JL. The etiology of malocclusion and the scientific method. Orthodontic Practice US. 2020; 11(2):62-65.
- Hayes JL. A new regimen of phase I care applied to anterior open bite — 10 case studies: an etiology proposed by the strategy of triangulation. Orthodontic Practice US. 2012; 3(3):18-26.
- Hayes JL. The Williamsport Orthodontic Study. 2005. A 10-year, phase-1 only study (abridged).
- Hayes JL. Kennewick Man. Chapter 10, Orthodontics. The Scientific Investigation of an Ancient American Skeleton. TAMU Press 2014; 207-211.
- Hayes JL. March 8, 2003. PAO Meeting, Philadelphia, PA. Presentation: A Clinical Approach to Identify Transverse Discrepancies.
- Hayes JL. In search of improved skeletal transverse diagnosis. Part II: A new measurement technique used on 114 consecutive untreated patients. Orthodontic Practice US. 2010; 1(4):34-39.
- Hayes JL. A new regimen of phase I care applied to potential canine impactions. Orthodontic Practice US. 2013; 4(3):44-51.
- Lima AC, de Oliveira Ruellas AC. Spontaneous Correction of CL II Malocclusion After Rapid Palatal expansion. Angle Orthod. 2003;73(6):745-752.
- Guest SS, McNamara JA Jr., Baccetti T, Franchi L. Improving CL II malocclusion as a side-effect of rapid maxillary expansion: A prospective clinical study. Am J Orthod Dentofacial Orthop. 2010;138(5):585-591.
- Skelly AC, Dettori JR, Brodt ED. Assessing bias: the importance of considering confounding. Evid Based Spine-Care J. 2012;;3(1):9-12.
- Berger VW, Sankoh AJ. Prognostic Variables in Clinical Trials. Methods and Applications of Statistics in Clinical Trials: Concepts, Principals, Trials, and Design. Wiley Online Library. Accessed November 3, 2022.
- Bhide A, Shah PS, Acharya G. A simplified guide to randomized controlled trials. Acta Obstet Gynecol Scand. 2018; 97(4):380-387.
- Moyers RE, Riolo ML, Guire KE,.Wainwright RL, Bookstein FL. Differential diagnosis of Class II malocclusions Part 1. Facial types associated with CL II malocclusions. Am J Orthod. 1980;78(5):477-494.
- Fidler BC, Artun J, Joondeph DR, Little RM. Long-term stability of Angle CL II Division 1 malocclusions with successful occlusal result at end of active treatment. Angle Orthod. 1995;107(3):276-285.
- Enlow DH, Hans MG. Essentials of Facial Growth. W.B. Saunders; 1996.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores

 John L. Hayes, DMD, MBA, received his dental degree from the Boston University, H.M. Goldman School of Graduate Dentistry and his orthodontic certificate from the University of Pennsylvania, School of Dental Medicine, Orthodontic Department where he is a Clinical Associate. He continues to research and lecture on the advantages of early interceptive treatment and on the etiology of malocclusions. Dr. Hayes is in private practice in Williamsport, Pennsylvania, with his wife, Sharon, who is also an orthodontist. He can be reached at jhayesortho@comcast.net.
John L. Hayes, DMD, MBA, received his dental degree from the Boston University, H.M. Goldman School of Graduate Dentistry and his orthodontic certificate from the University of Pennsylvania, School of Dental Medicine, Orthodontic Department where he is a Clinical Associate. He continues to research and lecture on the advantages of early interceptive treatment and on the etiology of malocclusions. Dr. Hayes is in private practice in Williamsport, Pennsylvania, with his wife, Sharon, who is also an orthodontist. He can be reached at jhayesortho@comcast.net.
