CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
The purpose of this article is to present a critical analysis of the literature to compare the efficacy of MAD use with CPAP therapy and focus on already established factors of treatment success that have been recognized in the literature.
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions with the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify the various symptoms of sleep-disordered breathing.
- Identify various therapies for sleep-disordered breathing.
- Identify the multi-system sleep tests that are used to diagnose sleep disorders.
- Realize the efficacy of both CPAP and MAD therapy on sleep-disordered breathing.
- Recognize some side effects of the various treatments.

Drs. George J. Cisneros and Oliver F. Nicolay together with Benjamin J. Goldstein compare the efficacy of oral appliance therapy and CPAP therapy
Introduction
Sleep-disordered breathing (SDB) is a pathological state in which there is periodic and recurrent snoring and obstructive sleep apnea (OSA).1 Individuals with untreated OSA have associated unfavorable health outcomes such as cardiovascular disease, stroke, hypertension, and atrial fibrillation. Patients also suffer from a decreased quality of life, daytime sleepiness, and an increased mortality rate.2 Continuous positive airway pressure (CPAP) therapy has been proven successful in treating OSA by improving the quality of life (QOL), the Apnea-Hypopnea Index (AHI), and oxygen saturation parameters.3 Despite these improvements, patient compliance and adherence to this treatment has been an issue. Patients either reject this treatment or partially comply with it. In fact, the benefits of CPAP treatment may be negated by poor patient compliance and acceptance.4 Recently, oral appliances have been quite popular in the medical and dental communities in treating OSA. Although CPAP therapy is still the “gold standard” in managing the condition, oral appliance therapy (OAT) has become an acceptable alternative for those patients suffering with mild to moderate OSA because of its ease of use and increased patient compliance. Generally, OAT can be divided into tongue-retaining devices and mandibular advancement devices. This critical analysis of the literature seeks to compare the efficacy of MAD use with CPAP therapy and will focus on already established factors of treatment success that have been recognized in the literature. A PubMed database search was used with the keywords: “Mandibular Advancement Devices,” “Continuous Positive Airway Pressure,” and “Obstructive Sleep Apnea.” No restrictions were placed on the filter search engine.
Apnea-Hypopnea Index (AHI) and oxygen saturation
Polysomnography (PSG) is a multi-system sleep test used to diagnose sleep disorders that monitors the patient’s breathing patterns, oxygen levels in the blood, heart rhythms, and limb movements. One of the numerous data sets provided by the PSG is a valuable measurement tool called the Apnea-Hypopnea Index (AHI). The AHI is a measure of the amount of apnea (a temporary cessation of breathing, especially during sleep) or hypopnea (abnormally slow or shallow breathing) events per hour of sleep.5 Mild OSA is a condition that can register less than 15 events per hour, while those with moderate OSA can have an AHI ranging from 15-30 events. Individuals with severe OSA have an AHI greater than 30.
A number of studies have documented that CPAP can significantly improve AHI when compared with MADs.3,6,7 A randomized controlled study by Lam, et al., evaluating the effectiveness of OAT versus CPAP in 101 patients with an average AHI of 21.4, noted a 90% reduction in AHI (to <5 events/hr) with CPAP and a 50% reduction using OAT over a 2-month period. They also reported a 4.5% increase in oxygen saturation when using CPAP therapy.6 In a more recent randomized controlled study by Phillips, et al., it was also noted that there was a statistical difference in the reduction of AHI by CPAP over MAD therapy. In their study, 108 participants with a mean AHI of 25.6 were selected to determine the efficacy of MAD versus CPAP therapy. After 1 month of therapy, there was an 83% average reduction in AHI when using CPAP versus a 58% reduction for those selected to participate using MAD therapy. The same study recorded a 3.5% statistically significant difference in the oxygen saturation between treatments.3 In the Gagnadoux and colleagues’ randomized controlled study, this pattern was also present as they also saw an enhanced reduction in AHI in patients using CPAP (73.2%) when compared with those using MAD (42.8 %).7 In fact, Ferguson and co-workers concluded in their study that patients undergoing CPAP therapy were 1.9 times more likely of achieving an AHI of 10 or less.8 In a recent systematic review and meta-analysis looking at 15 RCTs that included 491 CPAP patients and 481 patients receiving MAD therapy, it was found that OAT devices were successful in generating a significant reduction in AHI, whereas the reduction of nightly events were 6.24 times greater with CPAP therapy. This same systematic review evaluated the oxygen saturation in 346 patients that received MAD devices versus 354 patients undergoing CPAP therapy. The authors reported that there was a 3.11% difference in the oxygen levels set forth by both treatment modalities in favor of CPAP.9 The largest oxygen saturation difference of 11.9% in favor of CPAP was found in a prospective cohort study by Ferguson, et al.8
The above reports strongly suggest that CPAP therapy is more effective than MAD therapy in reducing the AHI index while it increases oxygen saturation levels in both mild and moderate OSA patients.
Figure 1A: Oral Appliance Therapy (OAT): Mandibular Advancement Devices (MAD) – The top part of Figure 1A illustrates how Mandibular Advancement Devices (MAD) work by moving the mandible forward during sleep, thus increasing the size of the airway and reducing airway resistance. The amount of advancement needs to be carefully titrated over time to effect an improvement and varies for each individual being treated. The lower half of the above figure shows only four examples of the more than 100 MADs currently available
Quality of life (QOL)
The World Health Organization defines health as “a state of complete physical, mental, and social well-being and not merely the absence of disease.”10 It can follow from here that the measure of health is not only the measure of the frequency and the severity of the disease process but also a measurement in the quality of the life (QOL) that follows with that pathological state. OSA and its treatments can be assessed using various subjective questionnaires to evaluate the QOL. Such questionnaires include the Epworth Sleepiness Scale (ESS), the Functional Outcomes of Sleep Questionnaire (FOSQ), and the Item Short Form Health Survey (SF-36).
Doff, et al., compared the efficacy of CPAP versus MADs in 104 patients over a 2-year time period. Using a randomized controlled format with 52 patients in the CPAP group and 52 patients in the MAD group, they assessed subjective improvements in sleepiness (ESS), functional status (FOSQ), and health perceptions (SF-36) — each measured at yearly intervals. They observed no significant differences in improvement between both treatment groups, underscoring the therapeutic potential using either option.5 In another randomized controlled trial, Lam, et al., compared the efficacy of both treatment modalities using 101 mild to moderate OSA patients to assess the QOL using the Sleep Apnea Quality of Life Index (SAQLI). Both the MAD and CPAP appliances improved QOL, but as before, no significant difference was found between the two therapeutic approaches.6 Barnes, et al., in another randomized study, also found that both treatment devices were equally effective in 110 mild to moderate sleep apnea patients using the ESS and SF-36 scales. Moreover, they also reported no statistical difference in overnight PSG results.11 And lastly, Hoekema and colleagues looking this time specifically at more severe OSA patients (moderate and severe) found an improvement in FOSQ in both appliance types (13.7 ± 3.1 to 16.6 ± 2.8 for MADs and 13.9 ± 3.7 to 16.7 ± 3.1 with CPAP therapy). These investigators also found a reduction in ESS with a 12.9 ± 5.6 at baseline to 6.9 ± 5.5 following treatment with an OAT and found a comparable reduction with CPAP therapy (14.2 ± 5.6 to 5.9 ± 4.8),yielding no statistical difference between treatment modalities.12
Systematic reviews looking at QOL criteria have also found similar outcomes. In a 2015 systematic review that looked at 10 RCTs specifically looking at daytime sleepiness found an insignificant .08% increase with CPAP treatment compared to MAD.9 A recent systematic review and meta-analysis done on four RCTs found that MADs were equivalent to CPAP when improving the quality of life in patients with mild to moderate sleep apnea. A non-significant improvement of 2.18% was found in CPAP, and the two methodologies were still considered equivalent and efficient in improving subjective sleep parameters.9
One can see that both CPAP and MAD therapy can be useful methods to improve QOL measurements as the research suggests that both produce equivalent outcomes. By using either treatment modality, patients can improve their cognitive, social, and physical well-being.
Arousal Index and Sleep Architecture
Arousal Indices and Sleep Architecture measurements can also be used for comparing these two treatment modalities. The Arousal Index measures how many arousals a patient has per hour, or in other words, how many sleep disturbances a patient has per hour. Sleep Architecture is the measure of rapid eye movement (REM) during sleep. These are important measurements as they help to quantify an objective calibration as to the quality of sleep experienced.
A recent systematic review evaluating six RCTs with a total of 274 patients using MADs and 272 patients treated with CPAP noted that the latter had an overall significant mean reduction of 3.57 events/hr more than those treated with MAD.9 Barnes, et al., reported higher mean reduction of 5.50 arousals/hr, again suggesting that CPAP was more effective than an oral device.13
However, a number of other studies did not show any difference in the Arousal Index. Phillips, et al.,3 Aarab. et al.,14 and Randerath, et al.,15 all showed no significant differences between CPAP and MADs with regards to the Arousal Index. With respect to the percentage of REM sleep, a systematic review was completed looking at eight RCTs with 244 patients in the CPAP group and 244 in the MAD group. A meta-analysis showed no significant difference between the two treatment groups, showing that although they both increased the amount of REM sleep, one did not do better than the other.
From the preceding reports, it is suggestive that both treatments for OSA can have a significant effect on sleep efficiency. By using either treatment approaches for OSA, patients can better their chances for having a more productive and less interrupted sleep.
Hypertension
One of the biggest concerns with having untreated OSA is cardiovascular disease. The most measured cardiovascular outcome in OSA studies has been blood pressure.
A systematic review on multiple RCTs on the effectiveness of MADs lowering BP was completed. A weighted average was calculated based on the included papers. The mean reduction in systolic BP was 2.09 mmHg and 3.15 mmHg in diastolic pressure. Another meta-analysis completed stated that there was no significant difference in the reduction of BP with either CPAP or MAD therapy.9 Although there may not be a difference in the effectiveness between these treatment modalities, it has been suggested in the literature that a reduction of 2 mmHg may have long-term benefits by reducing cardiovascular risk.16 Similarly, there have been many other studies that have reported no reduction in the BP with either treatment modality. Trzepizur, et al.,17 and Phillips, et al.,3 reported no significant changes in posttreatment BP measurements with either CPAP or any other oral device.
Whether there is a reduction or not, it is clear that either OSA treatment devices has very little impact on BP measurements, and hypertensive patients should be monitored and controlled by conventional methods as supervised by their physician.
Figure 1B: OAT – Tongue Retaining Device (TRD) – In this appliance, the tongue goes into the anterior bulb. Pushing the tongue forward and giving the bulb a little squeeze create a suction that holds the tongue in a forward position. It is a lab-fabricated appliance and is made out of a flexible polyvinyl material
Side effects
Follow-up with patients during or after a study gives us crucial information regarding the various treatments. We can learn about compliance, adherence, and side effects of the proposed treatment plan. In OSA, the clinician needs to assess the patient for signs and symptoms of improving or worsening OSA.
Side effects of any oral appliance type include sore gums, sore teeth, increased salivation, TMJ discomfort, difficulty with chewing in the morning, and changes in occlusion.<sup.6,18 Side effects common with CPAP machines included dry mouth and throat, throat and nasal irritation, feelings of suffocation, rhinitis, claustrophobia, noise bothering, and facial irritation.8 Doff, et al., did make the point that patients should expect to see craniofacial changes, such as mandibular protrusion, with the continued use of MADs for more than 2 years.5 Interestingly, Tsuda, et al., assessed the craniofacial changes in adult subjects with OSA and found that use of nasal CPAP for greater than 2 years resulted in a significant retrusion of the anterior maxilla, a retroclination of maxillary incisors, a retrusive position of both B point and chin, a decrease in maxillary-mandibular discrepancy, and a decrease of convexity in the facial profile.21
A meta-analysis evaluating eight RCTs on the discontinuation rates due to side effects of MADs versus CPAP documented that the overall odds of discontinuing OSA therapy due to the chronic use of a MAD versus CPAP machine were 0.54:1.9 This would suggest that the respective side effects that would lead to discontinuation were more pronounced with the use a CPAP machine. Therefore, the evidence suggests that the frequency and severity of the side effects in CPAP tended to be more of an obstruction for patients than they were with the use of MADs.
Figure 2: Continuous Positive Airway Pressure (CPAP) machine – The top half of the figure above illustrates the use of the (CPAP) machine; the lower half shows a nasal CPAP on the left and a full face CPAP mask on the right and one of the most commonly used CPAP machines in the middle. A CPAP machine is prescribed by a physician and delivers just enough air pressure to a mask to keep upper airway passages open, preventing snoring and sleep apnea. The precise amount of air is carefully titrated and tested during a PSG study
Adherence and preference
Like many other disease processes, the success of OSA treatment is dependent upon patient compliance and adherence to the prescribed treatment regimens.
Barnes, et al., reported in a randomized control trial of 110 mild to moderate OSA patients that the average usage for CPAP machines was 3.6 hours per night. This measurement was taken objectively through a meter that clocked time and pressure. The MAD group kept a diary, and the average time spent with the oral device was 5.3 hours per night.13 Lam, et al., conducted a randomized control trial with 101 mild to moderate OSA patients. They found the average metered-clock time with the CPAP machine was 4.2 hours per night. This compared to a self-reported usage of 5.2 hours per night with the MAD appliance.6 Ferguson, et al.,8 Clark, et al,19 and Randerath, et al.,15 all reported that although there were side effects with each treatment, patients overwhelmingly chose MADs over CPAP for the long-term management of OSA.
Furthermore, patients were surveyed, and the majority felt that MADs were user-friendlier than CPAP machines. Phillips, et al., reported in a RCT that there was a 51% preference rate for MADs versus a 21.3% preference rate for the CPAP machine. It was also reported that measured compliance rate for CPAP in 126 patients with mild to moderate OSA was 5.2 hours per night. This compared to a larger compliance rate of 6.5 hours per night with MADs.3 Dieltjens, et al., reported a discontinuation rate of 8.8% after a 1-year follow-up with MADs. This compared to a variable 20%-50% discontinuation rate of CPAP after 1-year follow-up. They also reported an average compliance rate of 5.6 hours per night with MADs and a 5.2 hour per night with CPAP therapy.20 These nightly rates are consistent with the other studies of their kind.
Lastly, a systematic review conducted looking at 11 RCTs concluded that the MADs had better overall adherence, with a mean subjective adherence rate of .70 hours of compliance over CPAP. Three of the 11 RCTs included had adherence rates that were more than an hour more than CPAP. Moreover, seven of those RCTs indicated that the future use of MADs would be more prevalent than CPAP.9
It is clear from the literature that there is a preference for oral devices over the CPAP machine alternative. Over the years, it has been maintained in the area of sleep medicine that CPAP was the primary choice for OSA therapy; however, healthcare providers may need to keep in mind that the MADs may show superior results for some patients due to the higher satisfaction and compliance ratings.
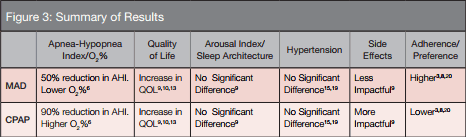
Figure 3: Above is a brief review of the data. CPAP and MAD therapy both are viable treatment options when treating mild to moderate obstructive sleep apnea. Although CPAP therapy may have a more dramatic effect on AHI (90% reduction) and O2 parameters, MADs are comparable to conventional CPAP machines in improvement in QOL indices. It is interesting to note that the side effects of CPAP machines were more of a hindrance to successful therapy. When discussing treatment with patients, the clinician should keep in mind the longer patient adherence and higher self-reported preference to oral appliance therapy.
Conclusion
For some time now, CPAP therapy has been the gold standard in the treatment of OSA. However, despite its therapeutic advantages, adherence and compliance are known problems. Many patients simply stop using the CPAP machine over time. Health outcomes and QOL indices were similar in patients with mild to moderate OSA for both treatment modalities. While CPAP may have the ability to enhance AHI and oxygen saturation parameters, this benefit could be negated by its poor compliance and adherence. And just as orthodontists do throughout treatment, it is recommended that all treating and supervising clinicians should be conducting rigorous follow-up throughout the therapeutic course to evaluate the evolution of OSA symptoms, with the added benefits of collecting a wealth of information available from doing so. Perhaps patient lifestyles and social preferences and preconceptions should be more thoroughly assessed by clinicians before prescribing a specific therapy since studies tend to suggest a preference for MAD over CPAP. This perspective could even potentially challenge the current practice parameters that tend to limit the usage of MADs to only mild to moderate OSA cases. In time, it could also lead to the possible usage of MADs as a primary treatment modality. With the advent of titratable and customizable oral devices, many more patients might end up preferring such devices for the long-term management of OSA. This developing trend in the area of sleep medicine challenges us as clinicians to be aware and prepared for the demand for our expertise. So as the role of the oral healthcare provider has become more relevant to the field of sleep-disordered breathing, we also need to be absolutely focused on the necessity for us to take part in the team approach for the successful treatment of OSA.
References
- Young T, Palta M ,Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836.
- Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887.
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with sleep apnoea/hypopnoea syndrome. Thorax. 1994;49(3):263–266,
- Doff MH, Hoekema A, Wijkstra PJ, et al. Oral Appliance Versus Continuous Positive Airway Pressure in Obstructive Sleep Apnea Syndrome: A 2-Year Follow-up. SLEEP. 2013;36(9):1289–1296.
- Lam B, Sam K, Mok WYW, et al. Randomized study of three non-surgical treatments in mild to moderate obstructive sleep apnoea, Thorax. 2007;62(4):354–359.
- Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34(4):914–920.
- Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. 1997;52(4):362–368.
- Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Sherene M. Thomas, Ph , Chervin RD. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J Clin Sleep Med. 2015;11(7):773-827.
- Measuring the quality of life. World Health Organization. WHO/MSA/MNH/PSF/97.4. Page 1 1997.
- Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664
- Hoekema A, Voors AA, Wijkstra PJ, et al.. Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. International Journal of Cardiology. 2008;128(2):232–239.
- Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664.
- Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration. 2011;81(5):411–419.
- Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002;122(2):569–575.
- Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomized trials. Lancet. 2003;362(9395):1527–1535.
- Trzepizur W, Gagnadoux F, Abraham P, et al. Microvascular endothelial function in obstructive sleep apnea: impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10(7):746–752.
- Holley AB, Lettieri CJ, Shah AA. Efficacy of an adjustable oral appliance and comparison with continuous positive airway pressure for the treatment of obstructive sleep apnea syndrome. Chest. 2011;140:1511–1516.
- Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie PA. crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109(6):1477–1483.
- Dieltjens M, Braem MJ, Vroegop AV, et AL. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502.
- Tsuda H, Almeida FR, Tsuda T, Moritsuchi Y, Lowe AA. Craniofacial changes after 2 years of nasal continuous positive airway pressure use in patients with obstructive sleep apnea. Chest. 2010;138(4):870–874.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores

 George J. Cisneros, DMD, MSc, received his BS from Manhattan College, DMD from the University of Pennsylvania School of Dental Medicine, and MMSc from Harvard University School of Dental Medicine. He is a Professor of Orthodontics at New York University College of Dentistry and is a Diplomate of the American Board of Pediatric Dentistry and the American Board of Orthodontics, and is a board examiner for both. Dr. Cisneros is a reviewer for various journals, including the American Journal of Orthodontics and Dentofacial Orthopedics, the Angle Orthodontist, the Journal of Dentistry for Children, and the Journal of Pediatric Dentistry where he also served as a member of the editorial board.
George J. Cisneros, DMD, MSc, received his BS from Manhattan College, DMD from the University of Pennsylvania School of Dental Medicine, and MMSc from Harvard University School of Dental Medicine. He is a Professor of Orthodontics at New York University College of Dentistry and is a Diplomate of the American Board of Pediatric Dentistry and the American Board of Orthodontics, and is a board examiner for both. Dr. Cisneros is a reviewer for various journals, including the American Journal of Orthodontics and Dentofacial Orthopedics, the Angle Orthodontist, the Journal of Dentistry for Children, and the Journal of Pediatric Dentistry where he also served as a member of the editorial board. Olivier F. Nicolay, DDS, MMSc, is Chair and Clinical Associate Professor – Department of Orthodontics at NYU College of Dentistry. He has a DCD from the Universite Paris Descartes in France, a DDS from Columbia University, and a Certificate in Orthodontics, Masters in Medical Sciences from Harvard. After graduation, Dr. Nicolay joined The Ohio State University where he taught postgraduate students and was involved in research. In 1989, he assumed the position of Program Director at Columbia University, pursuing his interests in research and teaching orthodontics. He has been member of the NYU College of Dentistry since 2002. He is a Diplomate of the American Board of Orthodontics, a Member of Angle East, component of the Angle Society of Orthodontists, the American Association of Orthodontists, and the American Dental Association.
Olivier F. Nicolay, DDS, MMSc, is Chair and Clinical Associate Professor – Department of Orthodontics at NYU College of Dentistry. He has a DCD from the Universite Paris Descartes in France, a DDS from Columbia University, and a Certificate in Orthodontics, Masters in Medical Sciences from Harvard. After graduation, Dr. Nicolay joined The Ohio State University where he taught postgraduate students and was involved in research. In 1989, he assumed the position of Program Director at Columbia University, pursuing his interests in research and teaching orthodontics. He has been member of the NYU College of Dentistry since 2002. He is a Diplomate of the American Board of Orthodontics, a Member of Angle East, component of the Angle Society of Orthodontists, the American Association of Orthodontists, and the American Dental Association. Benjamin J. Goldstein is a 4th-year dental student at New York University. He is a DDS candidate for the graduating class of 2018. He has received honors from the OKU society for his academic achievements. Goldstein plans to pursue postgraduate education in orthodontics following his graduation from NYU.
Benjamin J. Goldstein is a 4th-year dental student at New York University. He is a DDS candidate for the graduating class of 2018. He has received honors from the OKU society for his academic achievements. Goldstein plans to pursue postgraduate education in orthodontics following his graduation from NYU.
