 Comprehensive Technology and Clinical Improvements Continue to Support the Treatment of Orthodontic Patients
Comprehensive Technology and Clinical Improvements Continue to Support the Treatment of Orthodontic Patients
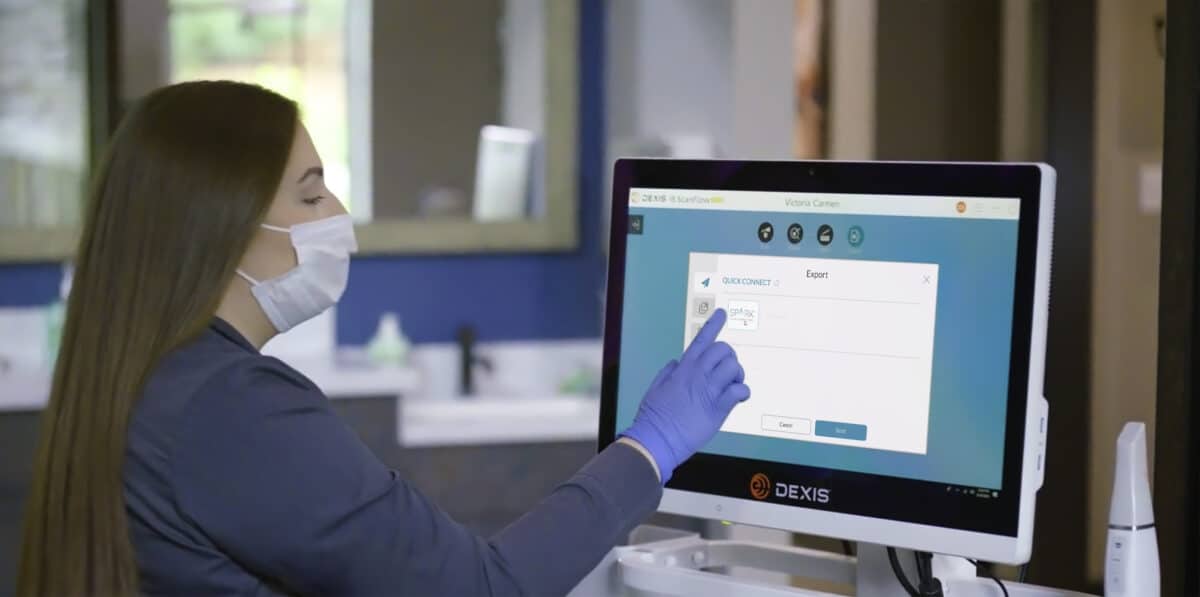
Brea, Calif. (Aug. 8, 2023) – Ormco Corporation announces the highly anticipated Spark Clear Aligners Release 14. Release 14 continues Spark’s philosophy of giving orthodontists control and flexibility. The Spark Aligner System is already an open platform that accepts scans from all major intraoral scanners. Now with Release 14, doctors will have even greater flexibility with the introduction of Spark Approver Web and seamless DEXIS™ IOS integration, along with exciting clinical and workflow updates.
“Spark Approver Web is more convenient to use, resulting in time savings for orthodontists by providing flexibility to access cases anytime, whether at the office or home,” said Eric Conley, President of Ormco. “This includes web-based access to the most commonly used desktop features of Spark Approver. In addition, DEXIS IOS integration optimizes Spark’s open platform by creating a continuous workflow from scanning to shipment.”

Spark Release 14 (R14) updates include:
- Using Spark Approver Web, cases can now be accessed and modified anywhere and on the go
- Work seamlessly between cloud-based and desktop-based Spark Approver Web
software - Secure file saving allows for data and information to stay saved on the cloud until they need to be accessed or modified
- No need for installation or updates
- Supported on commonly used internet browsers: Google Chrome, Microsoft Edge, Firefox and Opera
- Work seamlessly between cloud-based and desktop-based Spark Approver Web
- An optimized open platform through seamless integration of Spark Approver with DEXIS IOS resulting in a streamlined workflow
- Spark Approver open file platform is now directly integrated with DEXIS IOS ScanFlow, making case submission easier and faster
- Unlike the leading aligner brand, DEXIS IOS Solutions provides an open platform so practitioners can submit orders for functional appliances through their preferred provider.
“Spark Approver supports a more efficient workflow, with one-click ScanFlow exports, open file format, and more. The result is increased capabilities, performance, and productivity every step of the way,” said Dr. Alyssa Carter. “DEXIS IOS Solutions makes intraoral scanning simpler at every stage to seamlessly integrate scanning, planning, producing and treatment. Optimized efficiency, accuracy and flexibility in treatment planning is a true game-changer for my practice.”

Ormco also recently launched new clinical updates and user experience features, including:
Clinical Updates for Ultimate Control and Flexibility in Treatment Planning
- Spark’s Integrated Hooks now include three size choices in retention attachments. Spark’s Integrated Hooks deliver optimal retention and an enhanced patient experience.
- Now doctors can choose between TruGEN™ and TruGEN XR™ materials on all primary and refinement Spark product orders, including Spark 10, 20, and Advanced
- Both TruGEN and TruGEN XR have the same advanced force retention, clarity, stain resistance, and material thickness that orthodontists love
Enhanced Workflow Advancements
- Expanded Real-Time Approval allows for instant changes to improve efficiencies with Passive Aligner Editing. This update provides more flexibility when adding and editing passive aligners.
- TruRoot™ CBCT Collision Alert System predicts and prevents collisions during treatment to help doctors plan treatments with more precautions. This feature automatically detects and highlights areas on the root surfaces that are touching or penetrating cortical bone.
- Automatically import and align a CBCT scan with the TruRoot feature in Spark Approver from a local computer
- Aligner Trimline takes the guesswork out of your workflow with an accurate visualization of the actual trimline in the Spark Approver Software
- Web Scan Hold Resolution allows for faster workflow. Get detailed information in your dashboard to resolve scan issues with the new edit feature to minimize scan-matching errors.
For more information on Spark Release 14 and other recent improvements, please visit https://ormco.com/en-us/spark-r14-update or contact your Sales Representative.
About the Spark™ Clear Aligner System
Spark™ Aligners are manufactured by Ormco, a global leader in innovative orthodontics products, with 60 years of expertise, R & D and high manufacturing standards. Ormco has helped doctors treat more than 20 million patients in more than 140 countries. Spark Approver software is designed to give doctors more control and flexibility, while Spark’s advanced aligner technology and TruGEN™ material provide more sustained force retention and better surface contact with the tooth. The Spark Aligner is also designed to be clearer and more comfortable, and stains less than the leading aligner brand— which may be why 100% of patients recently surveyed said they would recommend Spark Aligners to a friend.*
For more information about Spark Aligners, visit https://ormco.com/en-us/spark
About Ormco
Envista is a global family of more than 30 trusted dental brands, including Nobel Biocare, Ormco, DEXIS, and Kerr, united by a shared purpose: to partner with professionals to improve lives. Ormco, headquartered in Brea, Calif., is a global leader and innovator of orthodontic products and solutions to help enhance the lives of its customers and their patients. For 60 years, Ormco has partnered with the orthodontic community to help create over 20 million smiles in over 140 countries. Distinguished products range from twin brackets (Symetri™ Clear, Titanium Orthos™ and Mini Diamond™) to pioneering self-ligating appliances with the Damon™ System (including Damon Ultima™ System and Damon™ Clear2). The Spark™ Clear Aligner System is designed to meet the needs of the orthodontist with the TruGEN™ material and Approver software. Ormco’s Insignia™ Advanced Smile Design™ provides an all-inclusive customized indirect bonding solution for efficiency through personalization. From personalized service to professional education programs and marketing support, Ormco is committed to helping orthodontists achieve their clinical and practice management objectives. Connect on Facebook at www.facebook.com/myormco and LinkedIn at www.linkedin.com/company/ormco.
*Data on file at Ormco
** The opinions expressed are those of Dr. Alyssa Carter as a paid consultant to Ormco. Ormco is a medical device manufacturer and does not dispense medical advice. Clinicians should use their own professional judgment in treating their patients. Individual patient results may vary
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores


