CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This self-instructional course for dentists aims to provide an overview of judicious use of antibiotics in the dental practice.
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions by taking the quiz online to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify bacterial pathogens implicated in dental
- Identify antibiotic treatment for specific dental infections.
- Analyze how to determine the drug-of-choice for certain treatments.
- Analyze prescribing indications related to duration of treatment.
- Recognize considerations related to the administration of medications for pediatric patients.

 Wiyanna K. Bruck, PharmD, and Jessica Price start their discussion on the judicious use of antibiotics in the dental practice
Wiyanna K. Bruck, PharmD, and Jessica Price start their discussion on the judicious use of antibiotics in the dental practice
Introduction
Antibiotics are one of the greatest medical advances since their first introduction in the late 1920s. Alexander Fleming’s breakthrough discovery of penicillin paved the way for a domain of medicine that has enabled once deadly infections to be readily treatable.1
Excessive antibiotic use comes with steep consequences of real-time adverse effects; downstream antibiotics resistance (also referred to as collateral damage); and superinfections, such as Clostridioides difficile. Overuse and inappropriate use of antibiotics has led to an increased prevalence of resistance, which translates to limited effectiveness of antibiotics, increased healthcare cost, and rising mortality rates. Antibiotic resistance is recognized as a global health threat. According to the Centers for Disease Control and Prevention (CDC), approximately 2 million Americans are infected with resistant pathogens that result in 23,000 deaths annually.2 The CDC conservatively approximates that 30% of all outpatient antibiotic prescriptions written from 2010 to 2011 were unnecessary.3 If the previous statistic was applied to the 2020 CDC data for number of antibiotic prescriptions written by dental practitioners in the United States, 7.29 million antibiotic prescriptions would be deemed as inappropriate within that 1 year.4
Judicious antimicrobial prescribing is essential in all fields of healthcare, including dentistry.5-7 The 5Ds of antimicrobial stewardship is a popular concept that should be applied to the appropriate prescribing of antibiotics. The 5Ds, include the right Drug, Dose, Delivery route, Duration of therapy, and De-escalation.8 Along with the concept of the 5Ds of optimal antimicrobial therapy, the CDC has constructed a Checklist for Antibiotic Prescribing in Dentistry (see Table 1), which serves as an excellent introduction to concepts that will be discussed in further detail.9

Bacterial pathogens implicated in dental infections
An odontogenic infection is a frequently encountered infection of the alveolus, jaws, or face that begins from a tooth or from its supporting structures. The most common cause of dental infections are dental caries, deep filling or failed root canal treatments, pericoronitis, and periodontal disease. The infection remains where it originates at the tooth or can spread into adjacent tissue or structures. The course of infection depends on several elements, including the virulence of the oral pathogens, the host resistance factors, and the surrounding anatomy.10
Determining the drug-of-choice for the treatment of odontogenic infections requires an understanding of which pathogens are implicated and whether antibiotics are truly necessary. Even when antibiotics are needed to treat dental infections, they might not be the first line therapy, but rather an adjunct after surgical drainage of an abscess, tissue debridement, or below the gum manipulation.
Bacteria that are frequently found in dental infections normally comprise the typical oral flora, which include a mixture of gram-positive streptococci (e.g., Streptococcus anginosus, Streptococcus mutans, Streptococcus intermedius group); anaerobic gram-negatives (e.g., Bacteroides spp, Prevotella spp., Fusobacterium); anaerobic gram-positives (e.g., Actinomyces spp., Peptostreptococci); and some rarer aerobic gram-negatives such as Eikenella corrodens. In general, the routinely used antibiotic agents (natural penicillin, aminopenicillin, penicillin combined with a β-lactamase inhibitor, and first-generation cephalosporins, tetracyclines, and macrolides) have good empiric coverage of the commonly implicated pathogens with the exception of coverage against anaerobic gram-negative bacilli.11-14 If there is a reason to suspect Prevotella species or other anaerobic gram-negative bacilli such as Fusobacterium or Bacteroides spp. that are positive for β lactamase (e.g., surveillance data suggest high prevalence), metronidazole is often added to the empiric coverage.5
Antibiotic treatment in dental infections
Pulpal pain, gingivitis, and periodontitis
Antibiotics are infrequently necessary for pulpal pain, gingivitis, and periodontitis. Irreversible pulpitis is characterized by acute and intense pain and is one of the most frequent reasons that patients seek emergency dental care. Antibiotics are not effective at treating pulpal pain, and treatment is not deemed appropriate if the patient has no signs of a spreading infection or systemic symptoms. Most important to note, inappropriate treatment of pulpal pain does not prevent the development of severe complications. Evidence suggests that combination therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen during meals and at bedtime is an effective way to manage pulpal pain.16 Aside from the removal of the tooth, the usual approach in the management of pulpitis is relieving the pain by drilling the tooth and removing the inflamed pulp and cleaning the root canal. There is no proof of benefit with using analgesics and or antibiotics in irreversible pulpitis.
Most patients with gingivitis or periodontitis can be effectively treated with mechanical debridement or scaling and root planing (SRP) without the need of antibiotic therapy. In patients that lack a response to SRP alone and have few sites of disease, a onetime local delivery of topical antibiotic can be utilized (e.g., chlorhexidine 2.5 mg chip, doxycycline 10% gel, minocycline 1 mg microsphere, or tetracycline 12.7 mg fiber). In patients with refractory cases that have extensive disease or those with severe or aggressive disease, strong evidence suggests adjunctive systemic antibiotics. Obtaining a culture to guide therapy prior to initiation is strongly encouraged if feasible.17-19
Abscesses
Most dental abscesses are secondary to dental caries and, therefore, can largely be prevented when basic and consistent preventative oral health measures are followed. Dental infections are common, including suppurative infections (abscesses), but not all need to be treated with antibiotics. It is therefore important to elucidate the presence of regional or hematologic spread suggesting disseminated infection and distinguish local versus systemic signs and symptoms of infection.20
Additionally, the treatment of abscesses depend on the location. If the dental abscess is localized, incision and drainage (I&D) alone is usually sufficient for proper recovery. In these cases, the patient will have a better outcome with a procedural I&D to obtain source control in comparison to the ineffective use of antibiotics alone. If the abscess is in the periapical region, treatment, including I&D, endodontic therapy, and adjunctive antibiotics are recommended.20-22 In patients who have severe systemic illness, in-patient treatment with intravenous antibiotics initially is generally justified.
If antibiotics are deemed necessary for either non-suppurative or suppurative odontogenic infections the agent selection is based on the coverage of oral typical oral pathogens. Historically, the drugs of choice were amoxicillin or penicillin V potassium. Although both agents were regarded as first line, amoxicillin was generally preferred due to having more robust gram-negative anaerobic coverage, less frequent dosing, ability to be taken on an empty stomach, and a lower incidence of gastrointestinal side effects.
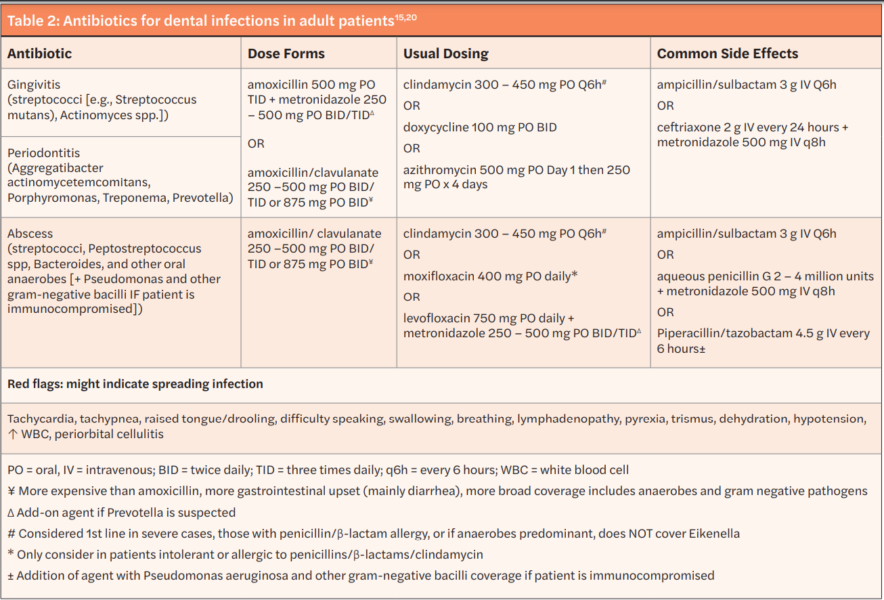
However, some experts recommend additional coverage of β-lactamase producing Prevotella and Fusobacterium with amoxicillin/clavulanate or a historic agent combined with metronidazole. Cephalexin — a first-generation cephalosporin with similar gram-positive aerobic coverage as the historic first line agents but that lacks robust anaerobic activity — is the antibiotic of choice in those with a history of penicillin allergy without history of anaphylaxis, angioedema, or hives. In patients with a severe allergy to penicillin agents or other β-lactams, azithromycin or clindamycin can be prescribed. When deciding on an agent to use for dental infection, consider azithromycin has higher rates of gram-positive aerobic resistance and clindamycin substantially increases the risk of developing a Clostridioides difficile infection but has much more robust coverage of mouth flora with the exception of Eikenella. Therefore, the risks and benefits of treatment in patients with a severe β-lactam allergy should be weighed prior to prescribing an alternative agent.20-23 A summary of antibiotics used for dental infections in adult patients as well as red flags for progressing infection are available in Table 2.
Duration of treatment
If treatment is initiated for non-suppurative indications (e.g., gingivitis and periodontitis), antibiotic therapy is usually until oral lesions have healed and pain has subsided, typically 5 to 7 days. Antibiotic duration of therapy for suppurative infections is usually 3 to 7 days depending on clinical improvement. The implementation of follow-up for patients initiated on antibiotic therapy is of utmost importance. Dentists should reevaluate patients for improvement or lack thereof with an in-person visit, telehealth appointment, or follow-up phone call. In general, most patients can stop taking antibiotics after 24 hours of complete symptom resolution, irrespective of re-evaluation after 3 days. If patient symptoms do not improve with initial therapy, clinicians should consider broadening therapy by either adding metronidazole to first- line therapy or discontinuing initial therapy and beginning amoxicillin/clavulanate (both options are typically for a 7-day duration).23
With adequate source control, short antibiotic courses have been found to be effective. A prospective clinical study was performed over a 3-year period with the objective to evaluate shortened courses of antibiotics in the management of dentoalveolar abscesses. After abscess drainage, patients were treated with amoxicillin, erythromycin, or clindamycin. A robust 98.6% of the 759 patients had full resolution of symptoms at day three of treatment, and antibiotics were discontinued at that time. The study concluded that the duration of antibiotics in most patients with acute dentoalveolar infections can safely be 2 to 3 days, provided I&D has been performed.26

Antibiotic treatment — pediatric considerations
The administration of medications for pediatric patients is even more complex compared to adults because of the need to dose based on the child’s weight and take into consideration concerns that are not applicable to adults. Adverse reactions caused by antibiotics are the most commonly reported reason for emergency department (ED) visits by those who are under the age of 18, which is represented by 140,000 ED visits each year specifically due to antibiotic adverse drug events.27
In addition, pediatric patients still have the same risks of side effects from antibiotics as adult patients, including the potential of increased resistance, allergic reactions, development of Clostridioides difficile infections, drug interactions, and common as well as rare side effects. Pediatric patients as young as 4 years old were found to harbor multidrug resistant bacteria in their mouths because of the overuse of antibiotics.28 Moreover, some antibiotics routinely used in adults have either not been studied in children or have demonstrated concern in animal models or in pediatric case reports. Tetracyclines and fluoroquinolones are two classes of antibiotics that are generally avoided in children.29 Tetracycline antibiotics (including doxycycline) may cause permanent tooth discoloration, enamel hypoplasia in developing teeth, and hyperpigmentation of the soft tissues. Because of the tooth and soft tissue related side effects of tetracyclines, their use is not recommended for pregnant women or children under the age of 8 years old. However, short-term (less than 21-day) use of doxycycline is advocated by the American Academy of Pediatrics as appropriate when benefits outweigh risks for certain infections due to the lack of evidence of the tooth discoloration side effect.30 Fluoroquinolone antibiotics appear at first glance to be an attractive choice since they are broad-spectrum agents, highly active in vitro against gram-positive and gram-negative pathogens, and are dosed only 1 to 2 times a day. However, these agents have numerous warnings associated with their use in patients of all ages as well as concerns related to increased possibility of musculoskeletal adverse effects in children.30 Fluoroquinolone (moxifloxacin) use within dentistry should be reserved for pediatric patients who are unable to take first- and-second line agents due to allergy or resistance.
When a dental clinician is considering antibiotic use in children, it is important as with adults to make sure that antibiotics are truly warranted.30-33 If caregivers are pressing for a prescription despite lack of evidence for necessity, it is wise to educate on the downstream negative effects. If antibiotics are considered appropriate, dosing and route of delivery should be taken into consideration as well as common and uncommon red flags for use in pediatrics (summarized in Table 3).33-41 Not only should the negative effects of antibiotics be discussed, but the prevention of dental caries with appropriate oral health maintenance should be reinforced.32
Summary
It is important to properly walk the fine line of treating infections that need to be managed with a short course of narrow spectrum antibiotics versus overprescribing antibiotics for either noninfectious indications or localized dental infections that don’t warrant antibiotics. With judicious use of antibiotics, dental practitioners can help curb the global threat of antibiotic resistance as well as avoid unnecessary side effects and increased cost for patients. Table 1 provides a summary of tools that can enable such practice.
Disclaimer
Clinician expertise in addition to evidence should be used when making treatment decisions for patients.
After reading about judicious antibiotic use, check out our CE on “Managing controlled substances in dental practice: prescribing and record keeping” for an overview of necessary considerations when prescribing and storing controlled substances. https://orthopracticeus.com/ce-articles/managing-controlled-substances-in-dental-practice-prescribing-and-record-keeping/
References
- Macfarlane G. Alexander Fleming: The Man and the Myth. Harvard University Press; 1984.
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; 2013.
- Fleming-Dutra KE, Hersh Al, Shapiro DJ, et al. Prevalence of inappropriate prescriptions among US ambulatory care visits, 2010-2011. 2016;315(17):1864-1873.
- Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions – United States, 2020.
- Fluent MT, Jacobsen PL, Hicks LA. Considerations for responsible antibiotic use in dentistry. J Am Dent Assoc. 206:147(8):683-686.
- Durkin MJ, Hsueh K, Haddy Y, et al. An evaluation of dental antibiotic prescribing practices in the United States. J Am Dent Assoc. 2017:148(12):878-886
- Ralph D, Azarpazhooh, Laghapur N, Suda KJ, Okunseri C. Role of dentists in prescribing opioid analgesics and antibiotics: an overview. Dent Clin North Am. 2018: 62(2):279-294.
- Joseph J, Rovold KA. The role of carbapenems in the treatment of severe nosocomial infections. Expert Opin Pharmacother. 2008;9(4):561-574.
- Centers for Disease Control and Prevention. Antibiotic Stewardship. https://www.cdc.gov/oralhealth/infectioncontrol/faqs/antibiotic-stewardship.html. Accessed June 23, 2022.
- Ogle OE. Odontogenic Infections. Dent Clin N Am. 2017(61):235-252.
- Dental and Periodontal Infections. In: Ryan KJ. eds. Sherris & Ryan’s Medical Microbiology, 8th edition. McGraw Hill; 2022. https://accessmedicine.mhmedical.com/content.aspx?bookid=3107§ionid=260928993. Accessed June 23, 2022.
- López-González E, Vitales-Noyola M, González-Amaro AM, et al. Aerobic and anaerobic microorganisms and antibiotic sensitivity of odontogenic maxillofacial infections. 2019 107(3):409-417
- Peterson LR, Thomson RB. Use of the clinical microbiology laboratory for the diagnosis and management of infectious diseases related to the oral cavity. Infect Dis Clin North Am. 1999;13(4):775-795
- Brook I, Frazier EH, Gher ME. Microbiology of periapical abscesses and associated maxillary sinusitis. J Periodontol. 1996;67(6):608-610.
- Tanner A, Stillman N. Oral and dental infections with anaerobic bacteria: clinical features, predominant pathogens, and treatment. Clin Infect Dis. 1993;16(suppl 4):S304-S309.
- Moore PA, ZieglerKM, Lipman RD, et al. Benefits and harms associated with analgesic medications used in the management of acute dental pain: an overview of systemic reviews. J Am Dent Assoc. 2018; 149(4):256-265.
- Santos RS, Macedo RF, Souza EA, et al. The use of systemic antibiotics in the treatment of refractory periodontitis: A systematic review. J Am Dent Assoc. 2016;147(7):577-585.
- Gonzalez JR, Harnack L, Schmitt-Corsitto G, et al. A novel approach to the use of subgingival controlled-release chlorhexidine delivery in chronic periodontitis: a randomized clinical trial. J Periodontol. 2011;82(8):1131-1139.
- Mylonas I. Antibiotic chemotherapy during pregnancy and lactation period: aspects for consideration. Arch Gynecol Obstet. 2011:283(1):7-18.
- Robertson DP, Keys W, Rautemaa-Richardson R, et al. Management of severe acute dental infections. 2015;350:h1300
- Jaramillo A, Arce RM, Herrera D, et al. Clinical and microbiological characterization of periodontal abscesses. J Clin Periodontol. 2005;32(12):1213-1218.
- Ahmad N, Abubaker AO, Laskin DM, Steffen D. The financial burden of hospitalization associated with odontogenic infections. J Oral Maxillofac Surg. 2013;71(4):656-658.
- Shukairy MK, Burmeister C, Ko AB, Craig JR. Recognizing odontogenic sinusitis. A national survey of otolaryngology chief residents. Am J Otolaryngol. 2020;41(6):102635.
- Jaworsky D, Reynolds S, Chow AW. Extracranial head and neck infections. Crit Care Clin. 2013;29(3):443-463.
- Zawislak E, Nowak R. Odontogenic head and neck region infections requiring hospitalization: An 18-month retrospective analysis. BioMed Res Int. 2021;708763.
- Martin MV, Longman LP, Hill JB, Hardy P. Acute dentoalveolar infections: an investigation of the duration of antibiotic therapy. Br Dent J. 1997;183(4):135-137.
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance Threats in the Unites States, 2013.
- Ready D, Bedi R, Spratt DA, Wilson M. Prevalence, proportions, and identities of antibiotic-resistance bacteria in oral microflora of healthy children. Micro Drug Resist. 2003;9(4):367-372.
- Jackson MA, Schutze GE, Committee On Infectious Diseases. The Use of Systemic and Topical Fluoroquinolones. 2016;138 (5):e20162706.
- Committee on Infectious Diseases, American Academy of Pediatrics. Antimicrobial agents and related therapy, Section 4. In: Kimberlin DW, Barnett ED, Lynfield R, et al. eds. Red Book: 2021-2024 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics; 2021.
- Goel D, Geol GK, Chaudhary S, Jain D. Antibiotic prescriptions in pediatric dentistry: A review. J Family Med Prim Care. 2020; 9(2):473-480.
- Fontana M, Karimbux NY, Cabezas C, Kim DM, Dragan IF. Dental Caries and Gingival and Periodontal Infections. In:. Pediatric Infectious Diseases: Essentials for Practice. Shah SS, Kemper AR, Ratner AJ (eds). McGraw Hill; 2019.
- American Academy of Pediatric Dentistry. Use of antibiotic therapy for pediatric dental patients. The Reference Manual of Pediatric Dentistry. American Academy of Pediatric Dentistry; 2021. American Academy of Pediatric Dentistry. Useful medications for oral conditions. The Reference Manual of Pediatric Dentistry. American Academy of Pediatric Dentistry; 2019.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp. https://online.lexi/com Lexicomp; 2022. Accessed June 23, 2022.
- Amoxicillin/Clavulanate. Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. . https://online.lexi/com. Lexicomp; 2022. Accessed June 23, 2022.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
- Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
- Penicillin V potassium. Pediatric and Neonatal Lexi-Drugs. Lexicomp Online. https://online.lexi/com. Accessed June 23, 2022.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores

 Wiyanna K. Bruck, PharmD, and Jessica Price start their discussion on the judicious use of antibiotics in the dental practice
Wiyanna K. Bruck, PharmD, and Jessica Price start their discussion on the judicious use of antibiotics in the dental practice Wiyanna K. Bruck, PharmD, BCPS, BCIDP, BCPPS, is an assistant professor of Pharmacy Practice at South College School of Pharmacy as well as an Antimicrobial Stewardship and Emergency Medicine Clinical Pharmacist practicing at a community hospital. She teaches infectious diseases as well a pediatric pharmacotherapy to both pharmacy and physician assistant students. Dr. Bruck received her bachelor of science degree in biology, followed by a Doctorate of pharmacy degree, then completed a postgraduate pharmacy residency program at William Beaumont Hospital in Troy, Michigan. Her research interests include antimicrobial stewardship, infectious diseases, as well as food allergy awareness. Dr. Bruck is Board-certified in pharmacotherapy, infectious diseases, and pediatrics.
Wiyanna K. Bruck, PharmD, BCPS, BCIDP, BCPPS, is an assistant professor of Pharmacy Practice at South College School of Pharmacy as well as an Antimicrobial Stewardship and Emergency Medicine Clinical Pharmacist practicing at a community hospital. She teaches infectious diseases as well a pediatric pharmacotherapy to both pharmacy and physician assistant students. Dr. Bruck received her bachelor of science degree in biology, followed by a Doctorate of pharmacy degree, then completed a postgraduate pharmacy residency program at William Beaumont Hospital in Troy, Michigan. Her research interests include antimicrobial stewardship, infectious diseases, as well as food allergy awareness. Dr. Bruck is Board-certified in pharmacotherapy, infectious diseases, and pediatrics. Jessica Price is a Doctor of Pharmacy candidate at South College School of Pharmacy in Knoxville, Tennessee. She has a Bachelor of Arts degree in Advertising and Public Relations, with minors in Business and English Writing from the University of Central Florida. Price completed her post-baccalaureate track in Biology at Florida International University and at the University of Tennessee, Knoxville.
Jessica Price is a Doctor of Pharmacy candidate at South College School of Pharmacy in Knoxville, Tennessee. She has a Bachelor of Arts degree in Advertising and Public Relations, with minors in Business and English Writing from the University of Central Florida. Price completed her post-baccalaureate track in Biology at Florida International University and at the University of Tennessee, Knoxville.
