CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This article aims to identify some of the changes in various age groups that result from mouth breathing to help dental professionals make informed decisions for guiding patients to improving their overall health.
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions on page 34 to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify airway and dental anatomy during various stages of life.
- Realize the benefits of nasal breathing.
- Realize the issues related to mouth breathing.
- Realize how the proper development of the craniofacial features is related to nasal breathing and mouth breathing.
- Recognize some possible treatments for adjusting breathing habits.

Michael Flanell, RDH, MBA, discusses mouth breathing and how clinicians can help patients make informed decisions regarding treatment
Introduction
From birth we are designed to breathe through the nose. Infants take their first breath through their noses once they are born. Newborn infants are considered obligate nasal breathers, hence dependent on a patent nasal airway for ventilation.25 This does not mean that under certain conditions, infants cannot breathe through their mouths.
Throughout life, there are conditions that can change a person from properly breathing through the nose to breathing through the mouth. The nose is the correct orifice to breathe through, and nasal breathing brings benefits for maintaining a person’s health that mouth breathing does not. Besides limiting a person’s ability to perform healthy functions, mouth breathing adversely affects the development of the craniofacial features and proper function of the dental occlusion as well as mandible and maxillary growth. This article will examine breathing during the different stages of life and summarize some of the deleterious effects of mouth breathing and the benefits of nasal breathing. The dental team is in an exceptional position to address these concerns with patients since they often observe the adverse effects of mouth breathing during routine dental visits. Identifying some of the changes that result from mouth breathing will help dental professionals guide their patients to make informed decisions for improving overall health for themselves as well as family members.
Infants from 1 month to 2 years old
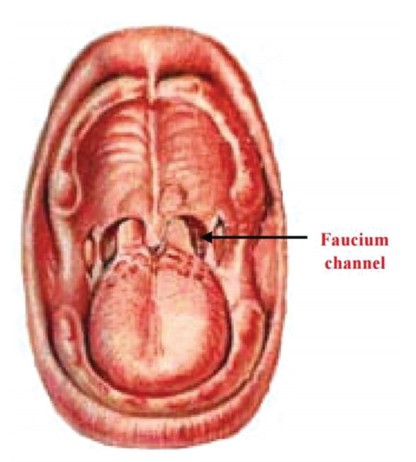
 Infants are born obligate nasal breathers depending on a patent nasal airway for ventilation.25 Newborns depend on nasal breathing to adapt to behavior competently in relation to ingestion and sucking. This specialization of oral behavior evolved in infants in response to breastfeeding.41 Anatomically, the oral airway of the infant is comparatively smaller than the airway of the adult (Figure 1). The epiglottis of an infant nearly touches the soft palate. The descent of the epiglottis is not complete until about 15 years of age, or when the vocal cords are fully matured.25 The infant’s tongue, in ratio to the oral cavity of the mouth, is larger than in the adult mouth, and the infant has an elongated, more rigid omega-shaped epiglottis and a smaller opening between the soft palate and the epiglottis.42 The position of the infant’s tongue is entirely within the oral cavity, allowing the distinctly omega-shaped epiglottis to interlock with the soft palate when breastfeeding. This forms a barrier, creating a straight route for air to travel from the nose to the lungs while breast milk flows through the faucium channels, thus allowing the infant to breathe and swallow simultaneously.42 This is an important feature for an infant’s growth and well-being as it optimizes the ability to nasal breathe and to take in nutrition concurrently.
Infants are born obligate nasal breathers depending on a patent nasal airway for ventilation.25 Newborns depend on nasal breathing to adapt to behavior competently in relation to ingestion and sucking. This specialization of oral behavior evolved in infants in response to breastfeeding.41 Anatomically, the oral airway of the infant is comparatively smaller than the airway of the adult (Figure 1). The epiglottis of an infant nearly touches the soft palate. The descent of the epiglottis is not complete until about 15 years of age, or when the vocal cords are fully matured.25 The infant’s tongue, in ratio to the oral cavity of the mouth, is larger than in the adult mouth, and the infant has an elongated, more rigid omega-shaped epiglottis and a smaller opening between the soft palate and the epiglottis.42 The position of the infant’s tongue is entirely within the oral cavity, allowing the distinctly omega-shaped epiglottis to interlock with the soft palate when breastfeeding. This forms a barrier, creating a straight route for air to travel from the nose to the lungs while breast milk flows through the faucium channels, thus allowing the infant to breathe and swallow simultaneously.42 This is an important feature for an infant’s growth and well-being as it optimizes the ability to nasal breathe and to take in nutrition concurrently.
 The proper development of the cranio-facial features has been attributed to breastfeeding and the simultaneous ability to breathe nasally. The jaw movements involved in extraction of milk from the breast provide major stimuli for growth of the temporomandibular joint and, consequently, encourage harmonious growth and development of the facial region. When performed correctly, nasal breathing also plays a role in the development of the maxilla and mandible to stabilize the dental occlusion, function, and muscle balance.15 Craniofacial development is largest within the first 4 years of life with 90% of development complete by 12 years of age.40 This proper formation is the beginning of a healthy craniofacial development that can support lifelong nasal breathing. Improperly formed craniofacial features can be a strong indicator of risk for the development of obstructive sleep apnea.16 The principal nongenetic determinant of maxillary growth is the route of breathing — nasal versus mouth breathing. Mouth breathing in infants results in a narrow maxilla, high-arched palate, and increased lower facial height and facial esthetics. This craniofacial pattern has been termed the “long-face syndrome” and is associated with obstructive sleep apnea (OSA). Thus, nasal obstruction causing mouth breathing affects maxillary growth and predisposes toward ensuing OSA. Infants with midfacial hypoplasia may develop life-threatening OSA in the first year of life.20
The proper development of the cranio-facial features has been attributed to breastfeeding and the simultaneous ability to breathe nasally. The jaw movements involved in extraction of milk from the breast provide major stimuli for growth of the temporomandibular joint and, consequently, encourage harmonious growth and development of the facial region. When performed correctly, nasal breathing also plays a role in the development of the maxilla and mandible to stabilize the dental occlusion, function, and muscle balance.15 Craniofacial development is largest within the first 4 years of life with 90% of development complete by 12 years of age.40 This proper formation is the beginning of a healthy craniofacial development that can support lifelong nasal breathing. Improperly formed craniofacial features can be a strong indicator of risk for the development of obstructive sleep apnea.16 The principal nongenetic determinant of maxillary growth is the route of breathing — nasal versus mouth breathing. Mouth breathing in infants results in a narrow maxilla, high-arched palate, and increased lower facial height and facial esthetics. This craniofacial pattern has been termed the “long-face syndrome” and is associated with obstructive sleep apnea (OSA). Thus, nasal obstruction causing mouth breathing affects maxillary growth and predisposes toward ensuing OSA. Infants with midfacial hypoplasia may develop life-threatening OSA in the first year of life.20
One common cause of mouth breathing and disruption of nasal breathing is the use of bottle feeding. Bottle feeding can separate the epiglottis/soft palate connection, elevate the soft palate, and drive the tongue back and down, altering the action of the tongue during rest, swallowing, speaking, and breathing. The infant then cannot suckle and breathe simultaneously, causing the gulping of liquids and the need to take in air through the mouth to sustain breathing while feeding. The lower the tongue, the smaller the pharyngeal airway. The largest oro-
pharyngeal airway is created when a person is breathing nasally with the lips sealed, and the dorsum of the tongue is as far forward as possible sealed against the hard and soft palate.25
When the mouth must adjust to an object other than the breast, the unnatural forces that develop can impact the shape of the palate.39 Weber, et al. (1986)43 noted that in breastfed babies, the tongue action appeared to be a rolling or peristaltic motion. However, the tongue action for bottle-fed babies was more piston-like or a squeezing motion. Picard (1959)35 wrote that in order to stop the abundant flow of milk from a bottle with an artificial nipple (with a large hole in the end), the infant was forced to hold the tongue up against the hole in the nipple to prevent the formula from gushing forth. This abnormal motor activity of the tongue is referred to as a tongue thrust or a deviate swallow. Due to downward placement of the tongue instead of palate placement, the floor of the nasal cavity rises as well. Since the bridge of the nose does not rise accordingly, there is a decrease in the total nasal space. This can have a dramatic effect on the individual’s breathing efficiency because the size of the nasal chamber is reduced. Studies have shown that a high palate and narrow arch are good predictors of snoring and obstructive sleep apnea.35 This eventually leads to the poor alignment of teeth and the “V-shaped” palate found in many people with malocclusions. This dynamic also explains how the upper back teeth are pulled inward to cause a mismatch or crossbite. Once a malocclusion develops, it can create a domino effect that can damage the rest of the teeth.40 Individuals with proper palatal placement of the tongue normally have a well-rounded and full “U-shaped” arch.
Excessive thumb-sucking can have the same impact on the oral cavity as bottle feeding. When the tongue is driven back by force, this can extend the distance between the soft palate and the epiglottis as well as block off the airway.23 The downward displacement of the mandible to keep the oral airways open encourages the start of oral breathing. Sasaki, et al. (1977),38 noted that “Age group 4 to 6 months seemed to represent a transitional period from obligate nasal breathing to potential oral tidal respiration. … This transition is important because it reflects a period of potential respiratory instability.”
Tongue-tie (ankyloglossia) is a condition present at birth that affects the oral development in restricting the tongue’s range of motion, affecting nursing/eating, speaking, swallowing, and breathing.29 Tongue-tie is the name given to the condition arising when the frenulum is unusually thick, tight, or short. There are many variations and differing degrees of severity.16 The tongue may not have the ability to rest in the palate, causing incorrect development of the palate/ nasal septum. The downward resting position of the tongue could then contribute to the development of mouth breathing. Nasal breathing benefits infants since the nose is the preferred route of breathing in infants because of its ability to humidify, warm, decontaminate, and regulate the air; thus, air reaches the lungs at the ideal temperature and favors oxygenation.4
Treatment for infants includes, but is not limited to, surgery for frenectomies, ENT evaluations to determine nasal obstructions, myofunctional therapy for teaching infants to keep a lip seal when not bottle feeding, adenotonsillectomy (T&A), and adenoidectomy.
Children from 2 to 12 years
Nasal breathing is associated with normal functions of chewing, swallowing, tongue posture, and lips, as well as providing correct muscular action that stimulates adequate facial growth and bone development.10 Mouth breathing (MB) is an etiological factor for sleep-disordered breathing (SDB) during childhood. The habit of breathing through the mouth may be perpetuated even after airway clearance. Both habit and obstruction may cause facial muscle imbalance and craniofacial changes.34 Mouth breathers demonstrated considerable backward and downward rotation of the mandible and increased overjet, causing an increase in the mandible plane angle, a higher palatal plane, and narrowing of both upper and lower arches at the level of canines and first molars when compared to the nasal breathers’ group. The prevalence of a posterior crossbite and an abnormal lip-to-tongue anterior oral seal was significantly more frequent in the mouth breathers’ group than with the nose breathers.17 Mouth breathing also causes dryness of the oral tissues, compromising gingival health. In addition, mouth breathing causes pathological changes in the nasopharyngeal and other respiratory tissues as well as muscle alterations, which influence deglutition, digestion, and phonation.9
In oral breathers, the chance of finding an asthmatic individual was almost 8 times greater than in the control group.37 This increased prevalence of asthma in oral breathers was already described in literature. This may be due to the fact of that there is a contiguous relationship between the upper and lower respiratory tract, beyond a higher prevalence of atopy in oral breathers. This way, the oral breathing allows the allergens, or the irritant agents, to reach the lower airways, causing bronchial hyperresponsiveness and asthma induced by exercise.37
The mouth-breathing syndrome (MBS), also known as long-face syndrome (Table 1), is the set of signs and symptoms of those who breathe partially or totally through the mouth. Felcar, et al. (2010),10 recognized that mouth breathing has multifactorial etiologies, such as hypertrophy of the palatine tonsils, adenoid hypertrophy, nasal septum deviation, nasal polyps, respiratory allergies, sinusitis, turbinate hypertrophy, sleeping position, and artificial feeding. He noted that mouth breathing may be caused by deleterious oral habits, such as digital sucking or pacifiers that, depending on the intensity and frequency, deform the dental arch and alter the whole facial balance. Some obvious facial features of mouth breathers are dark circles under the eyes, dry/cracked lips, head forward position, lips hanging open and flaccid, and constantly licking lips.
Mouth breathing is one of the most cited characteristics of sleep-disordered breathing (SDB) during childhood, but symptoms are often inadequately recognized. Pacheco, et al. (2015),33 stated that SDB encompasses a wide clinical spectrum, such as snoring, upper airway resistance syndrome (UARS), and obstructive sleep apnea (OSA). The authors noted that snoring during sleep was estimated to occur in between 8% and 27% of children, 2% of which present with OSA. Prevalence of UARS remains unknown and is most likely to be underdiagnosed. Findings for clinical diagnosis of UARS are considered nonspecific but strongly resemble clinical aspects of chronic mouth breathing and nasal obstruction.33 (See Table 1 for screening for MB.)
Mouth-breathing syndrome (MBS) is related to an adverse quality of life, especially as it refers to nasal problems, and sleep and eating concerns or difficulties.
Treatment for mouth breathers includes, but is not limited to, adenotonsillectomy, myofunctional therapy, allergy evaluation, airway centric-orthodontics, or a combination of any of these. It is important to consider a sleep evaluation pre- and post-therapy. Long-term follow-ups to treatments are recommended.
From teenage years through early adulthood
The most common cause of mouth breathing is the presence of obstacles in the nasopharyngeal region, which increases nasal resistance (decreases nasal airflow) that can be induced by various mechanical factors, including tonsil hyperplasia, hypertrophied turbinates, rhinitis, tumors, infectious or inflammatory diseases, and changes in nasal architecture. However, even after these mechanical factors are removed, MB continues in most cases due to the patient’s mouth breathing habit. Unbalanced facial musculature occurs as a result of MB, which causes changes in tooth positioning, lips, tongue, palate, and jaws, to counterbalance the new breathing pattern.34

Attention deficit hyperactivity disorder (ADHD) is commonly found among mouth breathers. When assessing for ADHD and complaints about school underachievement, da Costa, et al. (2015),9 found characteristics of snoring, nocturnal MB, rhinitis, tonsillitis, drool on the pillow, dark circles, and dry lips in more than half of their sample. Both ADHD and MB can trigger SDB, which, together with daytime sleepiness, directly interferes in school performance.
Based on the assumption of a possible relationship between body posture and breathing muscles, Okuro, et al. (2011),31 compared the maximal respiratory pressures (which are direct measures of respiratory strength) and head posture among mouth- and nasal-breathing children. The authors observed a decrease in maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) in mouth breathers, causing the formation of forward head posture (Figure 2) — the flexion of the lower cervical spine and extension of the upper cervical spine, which acts as a compensation mechanism for better performance of the respiratory muscle’s strength.21
Mouth breathers have difficulty in coordinating breathing and swallowing because they must stop chewing or chew the food faster to be able to breathe. Thus, the observed responses confirm the findings in another study by Canuto, et al. (2016),7 who found that the changes that occur in the respiratory pattern may lead to faster chewing: Chewing and swallowing take place in the same period of time that breathing occurs, causing the feeling of suffocation in the individual. However, the findings also indicate that most people consume liquids during meals while food is still in their mouths in order to ease the intake of solids, which aid in swallowing food faster and reducing the sensation of breathlessness and suffocation.
Saliva has many important functions. Garcia, et al. (2016),14 explained that saliva’s many functions are self-cleaning of the mouth, buffering and clearing acids, acquired pellicle formation, antimicrobial actions, and provision of ions for remineralization of demineralized enamel. Saliva protects the teeth from organic acids produced by bacteria that cause dental caries, and the extrinsic and intrinsic acids that initiate dental erosion. The depressed resting salivary flow is associated with lower plaque pH, increased numbers of lactobacilli and candida species, and greater caries risk. This could have serious consequences for caries activity and will also increase the risk of tooth loss via dental erosion.12 This study further explains how mouth breathing can cause water loss — a potential factor that could contribute to oral dryness.
Some studies have failed to find associations between mouth breathing and caries risk or salivary patterns. For example, Koga-Ito, et al. (2002),22 found no differences in caries risk between treated and untreated children for mouth-breathing syndrome, although the level of IgG antibodies to Streptococcus mutans (cariogenic bacteria) was higher in the treated group. Another study by Mummolo, et al. (2018),29 did not find differences in flow rates or buffering capacities of resting and stimulated saliva between mouth- and nose-breathing adolescents aged 10 to 19 years. However, Al-Awadi and AI-Casey (2013)3 found lower salivary flow rate among male patients 18 to 22 years old with mouth breathing associated with nasal obstruction in comparison to nose breathers. Mouth breathing was also associated with lower salivary pH, higher plaque index, and increased salivary S. mutans counts. Other studies also reported association between mouth breathing and dental caries. These findings are important since teenagers make more food choices outside the home, which usually include foods with a high sugar content and simple carbohydrates, which break down into glucose. These foods tend to be sticky, and the reduction in salvia to neutralize the pH and wash off debris from teeth increases the likelihood of promoting an environment ripe for dental cavities.
Some 13% of children aged 6 to 11 and 14% of adolescents aged 12 to 19 are overweight. The ever-increasing waistlines put children at risk for heart disease, Type 2 diabetes, and high blood pressure. But there is another problem, often overlooked, accompanying the grim statistics from the U.S. Surgeon General’s office. Those extra pounds around and in the oral pharyngeal also put children at risk for sleep apnea — a serious, debilitating and potentially life-threatening sleep disorder, according to the National Sleep Foundation.30 Sleep apnea symptoms include mouth breathing at night among other symptoms such as snoring, pauses in breaths while sleeping, difficulty getting up, behavior problems, and tiredness during the day.
Recommendations for care may include, but are not limited to, the following: myofunctional therapy, airway centric-orthodontia, administration of a sleep-test, CPAP, oral appliance therapy (if the patient is old enough), taping of the mouth, weight loss, dental examination, and oral homecare instructions.
Adults
 Increased upper airway resistance was associated with oral breathing during stable sleep, as compared with nasal breathing, irrespective of central or obstructive sleep apnea. Fitzpatrick, et al. (2003),13 confirmed that during sleep, upper airway resistance during oral breathing was 2.5 times higher than during nasal breathing. The route of breathing has a profound influence on upper airway resistance during sleep, with resistance being much greater during oral breathing than during nasal breathing.18 The substantially higher resistive load posed by the oral breathing route provides a plausible explanation for the observation that inhaled ventilation occurs almost exclusively via the nasal route during sleep in subjects with normal nasal resistance.11 Mouth opening has been shown to increase the propensity to upper airway collapse. Jaw opening is associated with a posterior movement of the angle of the jaw, which can compromise the oropharyngeal airway diameter. Also affected is the posterior and inferior movement of the mandible, which may shorten the upper airway dilator muscles located between the mandible and hyoid, compromising their contractile force by producing unfavorable length-tension relationships in these muscles.23
Increased upper airway resistance was associated with oral breathing during stable sleep, as compared with nasal breathing, irrespective of central or obstructive sleep apnea. Fitzpatrick, et al. (2003),13 confirmed that during sleep, upper airway resistance during oral breathing was 2.5 times higher than during nasal breathing. The route of breathing has a profound influence on upper airway resistance during sleep, with resistance being much greater during oral breathing than during nasal breathing.18 The substantially higher resistive load posed by the oral breathing route provides a plausible explanation for the observation that inhaled ventilation occurs almost exclusively via the nasal route during sleep in subjects with normal nasal resistance.11 Mouth opening has been shown to increase the propensity to upper airway collapse. Jaw opening is associated with a posterior movement of the angle of the jaw, which can compromise the oropharyngeal airway diameter. Also affected is the posterior and inferior movement of the mandible, which may shorten the upper airway dilator muscles located between the mandible and hyoid, compromising their contractile force by producing unfavorable length-tension relationships in these muscles.23
 Mouth breathing is also considered one of the predisposing factors for initiation of periodontal disease and/or its progression. The anterior dental open bite produced by chronic mouth breathing is associated with high incidence of periodontal disease and high risk of losing the anterior teeth in early ages, causing the absence of anterior guidance, which predisposes the patients for temporomandibular disorders (Table 2). The precise mechanisms are not fully understood, but probable causes are gingival surface dehydration, decreased epithelial resistance to bacterial plaques, and lack of salivary auto-cleaning.12 Mouth breathing at night dries the tissues, causing the mouth, teeth, tissues, and throat to be dry upon waking.
Mouth breathing is also considered one of the predisposing factors for initiation of periodontal disease and/or its progression. The anterior dental open bite produced by chronic mouth breathing is associated with high incidence of periodontal disease and high risk of losing the anterior teeth in early ages, causing the absence of anterior guidance, which predisposes the patients for temporomandibular disorders (Table 2). The precise mechanisms are not fully understood, but probable causes are gingival surface dehydration, decreased epithelial resistance to bacterial plaques, and lack of salivary auto-cleaning.12 Mouth breathing at night dries the tissues, causing the mouth, teeth, tissues, and throat to be dry upon waking.
 The forward head posture (Figure 2), common among mouth breathers, facilitates the air to enter the mouth which could lead to a deterioration of the pulmonary function. In the long run, the hyperactivity of the neck muscles may be associated with cervical changes that consequently can cause temporomandibular disorders (TMD) and cervical spine disorders. Considering all these aspects, a cycle seems to be established where mouth breathing alters the respiratory function and mechanics and produces postural compensations, which in turn perpetuate the respiratory changes.21 The low carbon dioxide levels associated with mouth breathing lead to overbreathing or hyperventilation. With less oxygen being delivered to the brain, muscles, and all the cells of the body, the body functions less than optimally.
The forward head posture (Figure 2), common among mouth breathers, facilitates the air to enter the mouth which could lead to a deterioration of the pulmonary function. In the long run, the hyperactivity of the neck muscles may be associated with cervical changes that consequently can cause temporomandibular disorders (TMD) and cervical spine disorders. Considering all these aspects, a cycle seems to be established where mouth breathing alters the respiratory function and mechanics and produces postural compensations, which in turn perpetuate the respiratory changes.21 The low carbon dioxide levels associated with mouth breathing lead to overbreathing or hyperventilation. With less oxygen being delivered to the brain, muscles, and all the cells of the body, the body functions less than optimally.
A large population of people became mouth breathers with the onset of sleep apnea. When an apnea episode occurs, a person stops breathing. As oxygen levels dip, the brain sends a signal to start breathing again, resulting in the loud snore and/or a sudden gasp to gulp in air. When an apnea event occurs during the night, the habit of sleeping with the mouth open can occur to accommodate the need for oxygen.31
Nose breathing is associated with many health benefits such as humidifying and cleansing/filtering the air to prepare it for the lungs. The structure of the nose is unique in that it regulates the direction and velocity of the airstream using turbinate’s maximizing exposure to the many arteries, veins, and lymphatics as well as to the nervous system and the mucous blanket. The turbinates also mix the air with nitric oxide, acting as vasodilators and increasing the opening of the veins for more oxygen/carbon dioxide exchange. Breathing through the nose increases the oxygen uptake ranging from 10 to 20 times greater when compared with mouth breathing. Finally, nasal breathing increases the circulating blood oxygen and carbon dioxide levels, slows the breathing rate, and improves overall lung function2 (Table 3).
Correcting mouth breathing in adults involves many of the same treatments as mentioned previously. Implementing myofunctional therapy is usually harder for adults than for children as children have a parent who motivates/prompts the therapy. Mouth taping at night may be easier in adults than in children as adults may not feel as “claustrophobic” as children. A thorough ENT evaluation should be included to determine if any nasal obstruction exists and should be completed before other treatments are considered. Adults may also need airway-centric orthodontics to correct malocclusion, which prevents the complete seal of the lips. Certain surgeries may be necessary as well. A sleep test should also be done to rule out and/or treat any sleep-breathing disorders prior to orthodontics, myofunctional therapy, or taping.
Senior Adults
Complaints of a dry mouth (xerostomia) and diminished salivary output (salivary hypofunction) are common in elderly people as a result of a plethora of salivary gland disorders, medication use, and medical disorders. Dry mouth problems have a clinically significant deleterious impact on oropharyngeal health. Clinicians must be able to diagnose dry mouth disorders in their elderly patients and provide preventive and interventional treatments to reduce the impact of these disorders on an older person’s quality of life. Mouth breathing in the elderly population can be an extreme contributor to the already debilitating effects of decreased salivary flow. Thus, it is especially helpful to identify if the patient is also contributing to these effects with mouth breathing, causing even more dryness. Establishing that a patient wakes up with a dry mouth may indicate the presence of a sleep-breathing disorder. Experiencing dry mouth upon awakening is a frequent symptom of OSA. Patients reporting dry mouth upon awakening have a 2.33-fold greater risk of having OSA rather than having primary snoring and are even at a higher risk of having severe OSA.33
Among patients with OSA, the collapsibility of the pharyngeal airway worsens with aging. Upper airway obstruction is caused by collapse of pharyngeal structures during sleep. It is known that mouth breathing increases upper airway collapsibility during sleep and may contribute to the occurrence of sleep-disordered breathing.33 The presence of hypertension, cardio-vascular disease, stroke, diabetes, and thyroid disease has been identified as factors that both result from and aggravate OSA.44
Mouth breathing in newly diagnosed sleep apnea patients is so prevalent that many sleep doctors start all their patients on CPAP therapy with a full-face mask. Full-face masks can be more challenging due to their sheer size. A larger mask frame and cushion create the greater possibility of leak simply because there is more surface area for potential leak. A mouth breather on CPAP has two choices — a full-face mask or a traditional nasal mask/nasal pillow mask with the addition of a chin strap.31 Patients with moderate-to-severe sleep-disordered breathing and a high percentage of mouth breathing during sleep were less adherent to CPAP therapy than patients exhibiting a low percentage of mouth breathing.3
Recommended treatments for the elderly include a sleep test to rule out a sleep breathing disorder, identifying if dry mouth is related to factors other than mouth breathing, ensuring that a nasal obstruction does not exist, and providing other therapies to support nasal breathing.
Conclusion
The dental care team should first understand that breathing was designed to be through the nose only. Many people lead their lives as mouth breathers without identifying why they experience certain changes in how they feel, how they look, or why they experience certain head and neck pains. Breathing is the first function for survival, and the body will do whatever it needs in order to support breathing, including prioritizing function over form. Thus, disfigurement of the face and neck may result from the body’s primary drive to inhale oxygen through whatever means it can. Dental teams that understand the functions and benefits of nasal breathing are empowered to help patients of all ages breathe and develop optimally. Identifying mouth breathing and why it is occurring in a patient are the first steps toward restoring proper breathing function and correcting anatomical formation. Sometimes it’s as easy as closing the mouth and starting to take in one breath at a time through
the nose.
References
- American Sleep Apnea Association. Sleep Apnea.org. https://www.sleepapnea.org/treat/cpap-therapy/troubleshooting-guide-for-cpap-problems/mouth-breathing-on-cpap/. Accessed June 15, 2020.
- American Sleep Apnea Association. Sleep Apnea.org. https://www.sleepapnea.org/treat/cpap-therapy/troubleshooting-guide-for-cpap-problems/mouth-breathing-on-cpap/. Accessed June 15, 2020.
- Al-Awadi RN, Al-Casey M. Oral health status, salivary physical properties and salivary Mutans Streptococci among a group of mouth breathing patients in comparison to nose breathing. J Bagh College Dentistry. 2013; 25(special issue 1):152-159.
- Allen The health benefits of nose breathing. Nursing in General Practice. 40-42. https://www.lenus.ie/bitstream/handle/10147/559021/JAN15Art7.pdf. Accessed June 10, 2020.
- Bachour A, Maasilta P. Mouth Breathing Compromises Adherence to Nasal Continuous Positive Airway Pressure Therapy. Chest Journal. 2004;126 (4):1248-1254. https://pubmed.ncbi.nlm.nih.gov/15486389/. Accessed June 10, 2020.
- Bergeson PS, Shaw JC. Are Infants Really Obligatory Nasal Breathers? Clin Pediatr. 2001;40(10):567-569. https://pubmed.ncbi.nlm.nih.gov/11681824/. Accessed June 10, 2020.
- Canuto MSB, Moura JB, Anjos CAL. Feeding preference of mouth breathers of an elementary school. Rev CEFAC. 2016; 18(4):811-817.
- Catalano P, Walker J. (06/2018) Understanding Nasal Breathing: The Key to Evaluating and Treating Sleep Disordered Breathing in Adults and Children. Department of Otolaryngology, St. Medical Center, Tufts University School of Medicine Medical, MA, USA. Gavin Publishers Lisle Illinois
- da Costa M, Valentim AF, Gonçalves Becker HM, Rodrigues Motta A. Findings of multiprofessional evaluation of mouth breathing children. Rev CEFAC. 2015;17(3):864-878.
- Crelin, ES. Development of the Upper Respiratory System. Clinical Symposia. 1976; 28(3).
- Farid MM, Metwalli N. Computed tomographic evaluation of mouth breathers among pediatric patients. Dentomaxillofac Radiol. 2010;39(1):1-10.
- Felcar JM, Bueno IR, Massan ACS, Torezan RP, Cardoso JR. [Prevalence of mouth breathing in children from an elementary school] [article in Portuguese]. Cien Saude Colet. 2010;15(2):437-444
- Fitzpatrick MF, McLean H, Urton AM, et al. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003;22:827-832.
- García Triana Elena Bárbara, Ali Ahlam Hibatulla, Ileana Bárbara, Grau León. Mouth breathing and its relationship to some oral and medical conditions: physiopathological mechanisms involved. Revista Habanera de Ciencias Médicas. 2016;15(2):200-212.
- Giugliani Justo PR, Caramez da Silva ER, Capsi Pires S. Influence of the duration of breastfeeding on quality of muscle function during mastication in preschoolers: a cohort study. BMC Public Health.2012;12(1):934.
- Grippaudo C, Paolantonio EG, Antonini G, et al. Association between oral habits, mouth breathing and malocclusion. Acta Otorhinolaryngol Ital. 2016;36(5):386-394.
- Guilleminault C, Parinen M, Hollman K, Powell N, Stoohs R. Familial aggregates in obstructive sleep apnea syndrome. Chest Journal. 1995:107(6):1545-155.
- Hall DMB, Renfrew MJ. Tongue tie. Arch Dis Child. 2005;90(12):1211-1215.
- Harari D, Redlich M, Miri S, Hamud T, Gross M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. 2010;120(10):2089-2093.
- Hollowell DE, Surratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol. 1991;71(6):2267-2273.
- Katz ES, Mitchell RB, D’Ambrosio CM (April 2010) Obstructive Sleep Apnea in Infants. American Journal of Respiratory and Critical Care Medicine. 185(8):805.
- Koga-Ito CY, Unterkircher CS, Watanabe H, et al. Caries Risk Tests and Salivary Levels of Immunoglobulins to Streptococcus mutans and Candida albicans in Mouth Breathing Syndrome Patients. Caries Res. 2003;37(1):38-43.
- Lopes Veron H, Antunes AG, Milanesi JDM, Corrêa ECR. Implications of mouth breathing on the pulmonary function and respiratory muscles. Rev CEFAC. 2016;8(1):242-251.
- Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691-707.
- Mayo Clinic. Tongue-tie. https://www.mayoclinic.org/diseases-conditions/tongue-tie/symptoms-causes/syc-20378452. Accessed June 15, 2020.
- Meurice JC, Marc GCI, Carrier G, Sériès F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996; 153:255-259.
- Miller MJ, Martin RJ. Carlo WA, et al. Oral breathing in newborn infants. J Pediatr. 1985;107(3)465-469.
- Moses AJ, Kalliath ET, Pacini G. Evolution of the Human Oral Airway. Dental Sleep Practice. 2017;(Winter):32-37 https://dentalsleeppractice.com/ce-articles/evolution-human-oral-airway-apnea//. Accessed June 15, 2020.
- Mummolo S, Nota A, Caruso S, et al. (2018). Salivary Markers and Microbial Flora in Mouth Breathing Late Adolescents. Biomed Res Int. https://pubmed.ncbi.nlm.nih.gov/29693018/?from_single_result=26.+Mummolo+S%2C+Nota+A%2C+Caruso+S%2C+et+al.+%282018%29.+Salivary+Markers+and+Microbial+Flora+in+Mouth+Breathing+Late+Adolescents.+Biomed+Res+Int. Accessed June 15, 2020.
- National Sleep Foundation: Sleep for Kids. Information about children’s sleep for Parents and Teachers. Children, Obesity, and Sleep. https://sleepforkids.org/html/obesity.html. Accessed June 15, 2020.
- Okuro RT, Morcillo AM, Sakano E, et al. Exercise capacity, respiration mechanics and posture in mouth breathers. Braz J Otorhinolaryngol. 2011;77(5):656-662.
- Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. Sleep Res. 2006;15(3):317-320.
- Pacheco MC, Casagrande CF, Teixeira LP, Finck NS, de Araújo MT. Guidelines proposal for clinical recognition of mouth breathing children. Dental Press J Orthod. 2015;20(4):39-44.
- Palmer B. The Influence of Breastfeeding on the Development of the Oral Cavity: A Commentary. J Hum Lact. 1998;14(2):93-98.
- Picard PJ. Bottle feeding as Preventive Orthodontics. J Calif State Dent Assoc. 1959;35:90-95.
- Popoaski C, Marcelino TF, Sakae TM, Schmitz LM, Correa LHL. Evaluation from the quality of life in the oral breathers patients. Int Arch Otorhinolaryngol. 2012;16(1):74-81.
- Fayez S, AIhamadi W. Orthosurgical Correction of Severe Vertical Maxillary Excess: Gummy Smile. Approaches to Orthodontics. 2018. https://www.intechopen.com/books/current-approaches-in-orthodontics/orthosurgical-correction-of-severe-vertical-maxillary-excess-gummy-smile. Accessed June 15, 2020.
- Sasaki CT, Levine PA, Laitman JT, Crelin ES Jr. et al. Postnatal Descent of the Epiglottis in Man. A Preliminary Report. Arch Otolaryngol. 1977;103(3):169-171.
- Shepard JW Jr, Gefter WB, Guilleminault C, et al. Evaluation of the Upper Airway in Patients with Obstructive Sleep Apnea. Sleep. 1991;14(4):361-371.
- Trabalon M, Schaal B. It takes a mouth to eat and a nose to breathe: abnormal oral respiration affects neonates’ oral competence and systemic adaptation. Int J Pediatr. https://www.hindawi.com/journals/ijpedi/2012/207605/. Accessed June 15, 2020.
- Tsui BCH. Physiological considerations related to the pediatric airway. Can J Anaesth. 2011;58:476-477.
- S. National Library of Medicine. Effect of Added Varnum Mouthpiece on Pharyngeal Collapsibility and Sleep Apnea Severity in Mouth Breathers. Published April 2016. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02738255. Accessed June 15, 2020.
- Weber F, Woolridge MW, Baum JD. An ultrasonographic study of the organization of sucking and swallowing by newborn infants. Dev Med Child Neurol. 1986;28:19-24.
- Wu JC, Dubois NMG. 2005. Role of Oral Devices in Managing Sleep-disordered Breathing Patients. Position Statement. American College of Prosthodontists: Chicago, IL. 2005.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores

 Michael Flanell, RDH, MBA, is a Certified Sleep Apnea clinician from the Academy of Clinical Sleep Disorders Disciplines, a Myofunctional Therapist, and a Breathing Coach. She is a professor to the Department of Healthcare Management at St. Joseph’s College in Patchogue, New York, where her responsibilities include designing and teaching healthcare administration online. Previously, she taught at Briarcliff College in the Dental Hygiene and Healthcare Management Department. Presently, she is an Operations Manager at Sleepwell Orthotics and a clinical consultant at Advanced Dental Sleep Consultants.
Michael Flanell, RDH, MBA, is a Certified Sleep Apnea clinician from the Academy of Clinical Sleep Disorders Disciplines, a Myofunctional Therapist, and a Breathing Coach. She is a professor to the Department of Healthcare Management at St. Joseph’s College in Patchogue, New York, where her responsibilities include designing and teaching healthcare administration online. Previously, she taught at Briarcliff College in the Dental Hygiene and Healthcare Management Department. Presently, she is an Operations Manager at Sleepwell Orthotics and a clinical consultant at Advanced Dental Sleep Consultants.
