CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This article aims to discuss possible ways in which the clinician can introduce and implement clinical and practice management systems to effectively deliver any orthodontic treatment in less time with decreased sensitivity.
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify the process of accelerated orthodontics.
- Realize some ways to introduce the concept to patients.
- Realize some of the history and clinical research behind accelerated orthodontics.
- Identify techniques regarding micro osteo-perforations.
- Identify uses for vibration for seating of aligners.

Dr. David Alpan discusses several technologies that deliver orthodontic treatment in less time
Abstract
Accelerated orthodontics (AO) historically has been associated with negative sequela, but with the use of bone modulation technology, it is now considered progressive. Reducing treatment time or increasing treatment predictability are the tangible benefits that are desired by patients and practitioners alike.22 Completing treatment early reduces possible risks and creates a more pleasant experience.17 Increasing predictability allows practitioners to treat more severe malocclusions in reasonable treatment times. Incorporating AO into practice requires changing treatment planning and practice management systems. Orthodontics creates change in the alveolar bone through biomechanics of applying force to teeth, but AO provides bone modulation affecting the biology of tooth movement on a cellular level. This article will describe my introduction, implementation, clinical, and practice management systems to effectively deliver any orthodontic treatment in less time with decreased sensitivity. The evolution of our profession will incorporate AO into the armamentarium of all orthodontists.
Introduction
“When will my treatment be completed?” Orthodontists are asked this question repeatedly. Eventually, I started to ask my patients, “When will your treatment be finished?” They always look at me funny and say, “You’re the doctor — you tell me.” My typical response is, “In a few more visits,” (which we know is probably not true). Orthodontists have a reputation of extending treatment in an effort to create perfect outcomes. The clinician’s dilemma is a wide variety of malocclusions, ethnicities, size of teeth, variable bone biology, and various levels of patient compliance. Predicting accurate treatment time is not easy, and many times, the answer is an estimate, based on our individual clinical experience. Unfortunately, no orthodontist can predict treatment length accurately 100% of the time. Research clearly demonstrates that accelerating the biology of tooth movement is a modality to add to our armamentarium.6,18,19,20,21
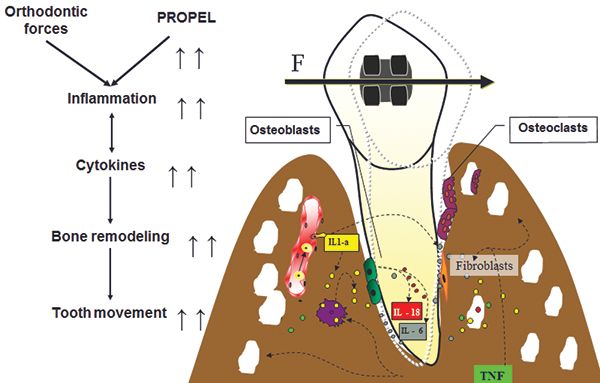
The practice of clinical orthodontics is managing the science of biomechanics, which inherently is harnessing the biology of tooth movement (Figure 1).1,7 My research project as a senior dental student in 1995 was on the influence of secondary messengers on calcium regulation in osteoblasts. I wanted to discover how orthodontists could influence the biology of tooth movement to reduce treatment time. My mentor and I were interested in finding the secondary messengers for calcium regulation, so we could influence the calcium regulation of osteoblasts and osteoclasts. We researched how to accelerate the biology of tooth movement, so we could create a drug to modulate the alveolar bone response. My research project was awarded first place from the ADA/Dentsply Student Research Competition in 1995 at University of the Pacific Dental School. I was invited to present this research at the Student Clinicians of American Dental Association (SCADA) at the 37th annual ADA convention in Las Vegas in 1995 as the sole representative from my dental school. We were searching to see which mechanism of increased calcium in the cell could regulate cyclic nucleotides in the arachidonic acid cascade. Our research found that the arachidonic acid cascade has no effect by cyclic nucleotides. Overall, stretch of the cell membrane was still the primary stimulator to increase calcium intracellular.22 Our goal was to isolate a messenger such as cytokines, find a stimulator, or create a drug that could attach to the proper receptor, so we could influence the biology or the rate of tooth movement.
In 1998, AO meant applying greater than 150 gm of force over less than 30 days. This concept was considered taboo, due to the negative sequela created (root resorption, bone loss, recession etc.). The main topics of discussion around acceleration were mechanical efficiency and friction. There was little research on the effect of trauma, sound, light, or vibration at that time. There was extensive literature in orthodontics comparing various biomechanics of appliances and their efficiencies regarding tooth movement. The introduction of biomechanical efficiencies such as prescription brackets, straight-wire mechanics, self-ligating versus non-self-ligating, clear brackets with various levels of friction, introduction of passive versus active self-ligation, lots of biomechanics with loops via Burstone mechanics, constant non-decaying force applications such as nickel-titanium wires, and nickel-titanium springs were the only forms of acceleration at that time. In 2016, AO now means having bone modulators (vibration and micro-osteoperforations) trigger the physiologic process of bone resorption and apposition at an increased rate. We also have even more efficient bracket systems using 3D modeling and planning to build custom brackets and wires to reduce treatment time with even greater levels of mechanical efficiency.
Research and clinical results
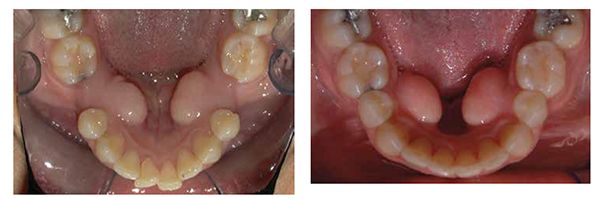
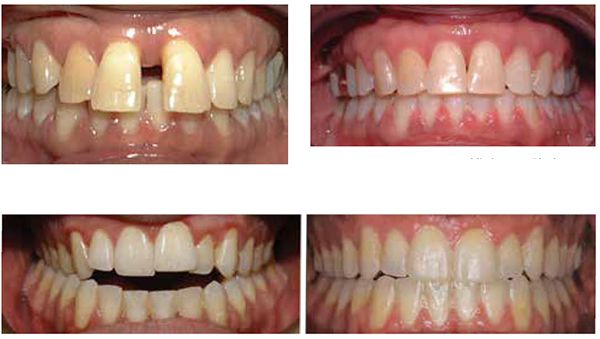
From 1988 to 2010, multiple researchers found that the application of NSAIDs decreased the rate of tooth movement significantly, and cytokines played an important role in activating the bone remodeling machinery.1,7,13 Other research showed that high forces created zones of necrosis through hyalinization. Thus optimal forces were considered to be lighter and more constant, facilitating efficient tooth movement with reduced or no sequela. As an early adopter of Damon® passive self-ligation (PSL), I saw the benefits of frictionless movement with lighter forces. Improved mechanical advantages and reduced treatment time lead me to believe PSL mechanics had accelerated tooth movement more efficiently than traditional metal twin brackets. Most of the PSL cases finished 6 months faster, but detailing was more challenging. Leveling and aligning became super easy, but the anterior/posterior (AP) and transverse corrections still took as much time to correct as traditional braces. As more research was conducted on AO, various products and techniques began to emerge. For example, using PSL in conjunction with TADs, NiTi closing springs, and three MOPs; we closed a 10 mm space with no sequela in 8 months (Figures 2-3).
Reduction in treatment time can now be directly controlled by choosing more efficient appliances (PSL, NiTi wires and springs, 3D setups, custom brackets), removing all NSAIDs, in combination with accelerating the biology of tooth movement via MOPs or with vibration. Research shows if inhibiting the expression of certain cytokines decreases the rate of tooth movement, then if we perform an iatrogenic trauma to stimulate the expression of inflammatory cytokines with MOPs, clinically we see an increase in the rate of tooth movement. Previous studies demonstrated bone injury causes cytokine release and leads to an accelerated bone turnover, as well as a decrease in regional bone density.2,3,4,5,8 The idea of traumatizing the bone is not novel and is now correlated with the increase in the inflammatory cytokines that can increase bone remodeling.6 Since MOPs were introduced with the use of TADs in 2009, a company called Propel® Orthodontics launched a patented tool to facilitate MOPs.
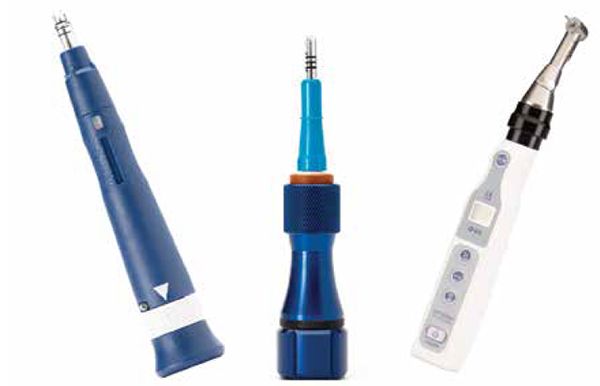
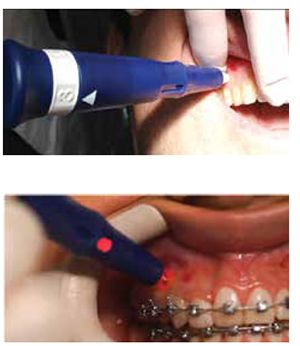
Propel is uniquely designed to perform MOPs to stimulate the alveolar bone to increase cytokines locally. The devices are FDA-registered 510(k) exempt Class I medical devices designed for single-use only (Figure 4). The first-generation Excellerator had a LED with a depth indicator and was disposable after one use. The device has a sleeve around the screw; when it is depressed, the screw penetrates into the bone. The pressure on the sleeve would trigger the light at the depth it was set to. The second-generation (Excellerator RT) has a reusable handle that can be sterilized with a disposable one-time use tip. There is no more LED, and depth can be measured with 3 mm markings on the sleeve, or a sleeveless tip is available. The third-generation Excellerator PT power tip is an automated slow speed driver with a contra-angle head attachment and single use burs with no measuring gauge. This device requires less time and effort, and offers improved access for the doctor to perform MOPs. Propel drivers are made with a surgical stainless-steel micro-leading edge designed and patented to be used to atraumatically perforate the alveolus directly through keratinized gingiva as well as movable mucosa. The Excellerators are designed to maximize the remodeling process, while reducing soft-tissue damage, and enable any orthodontist to perform MOPs easily chairside. We routinely perform this procedure as if we were placing a TAD or changing an archwire.
MOPs technique
Mild discomfort is experienced by the patient postoperatively, usually for 1 to 2 days, and is moderated with Tylenol® only, not NSAIDs.6,18,20,21 Some patients require the use of a local anesthetic via syringe, but in most cases, profound topical is sufficient. We start with benzocaine 20% for 3 to 5 minutes locally, then a compounded topical (lidocaine 10%, prilocaine 10%, and tetracaine 4%) for 30 to 60 seconds, followed by a MadaJet with lidocaine 2% 1:100,000 epinephrine spray. This is the same technique used for my TAD placements. The Propel technique is clearly outlined and explained in Nicozisis’ article.18 The decrease in number of visits leads doctors to be more profitable, independent of how they charge or do not charge for Propel.21 MOPs can be localized to two teeth whereas vibration affects all the teeth. When using MOPs, there is no issue of patient compliance, as there is with vibration. MOPs are performed every 12 to 16 weeks depending on the patient’s treatment response. When using MOPs reactively (during treatment), patients typically require 1 to 2 sessions. Those who want to reduce overall treatment time proactively (at the start) from the initial appointment may need 2 to 3 sessions. I create 2 to 3 MOPs interdentally vertically aligned from the crest, and I place the MOP driver 90 degrees to the tissue. The depth of perforation is usually 3 mm-5 mm. Patients’ soft tissue can vary in thickness from 1 mm-2.5 mm in thickness. So, the tip will go into the cortical plate and micro-fracture the cortical bone about 1.5 mm-3 mms in depth. Many patients can have the first and second MOP in attached tissue; the third MOP may be in the mucosal tissue.
Vibration technique
Since OrthoAccel publicly launched AcceleDent® Auran (Figure 19) in early 2012, clinicians and patients are experiencing reduction in treatment time23,24,25, increased treatment predictability, and an analgesic effect26. Micropulse vibration, 20 minutes per day at a frequency of 30 Hz at a force of 0.25N (25g) is seeing incredible results with accelerating tooth movement. The clinical research states 50% increase in the rate of tooth movement,6,23,24,25 but I have found this does not correlate to the same reduction in overall treatment time. Acceleration is more efficient during leveling and aligning then sagital corrections.24 With AO, I am seeing a clincal average of 35%-40% reduction in overall treatment time dependent on the appliance choice, mechanics utilized, or acceleration device used. AcceleDent Aura offers an analgesic effect26 which MOPs do not. The theory behind the effective mechanism of vibration is explained with an increased blood supply or the wiggle effect. Both reduces binding of the wire in the bracket (friction) or lack of tracking in aligners and allows for the biology of tooth movement to be more efficient. I strongly advise all patients using vibration not to use NSAIDs during treatment. To be efficient, and make AO work, doctors have to change patients appointment intervals. Patients that choose vibration need to be compliant daily: I check the compliance interface at each visit, which gives us a chronologic history by day, month, time and length of use. My Invisalign patients change aligners every 5-7 days, and I deliver 4 to 10 aligners per visit. Depending on how treatment is progressing, I may graduate to delivering more aligners at each visit or reduce the time in each aligner depending on progress. For 3M Incognito™, Insignia®/Damon, and mini twin mechanics we are activating treatment every 3 weeks for wire changes or repositioning bracket. During initial leveling, I am seeing results in one appointment that would normally take 3 visits.
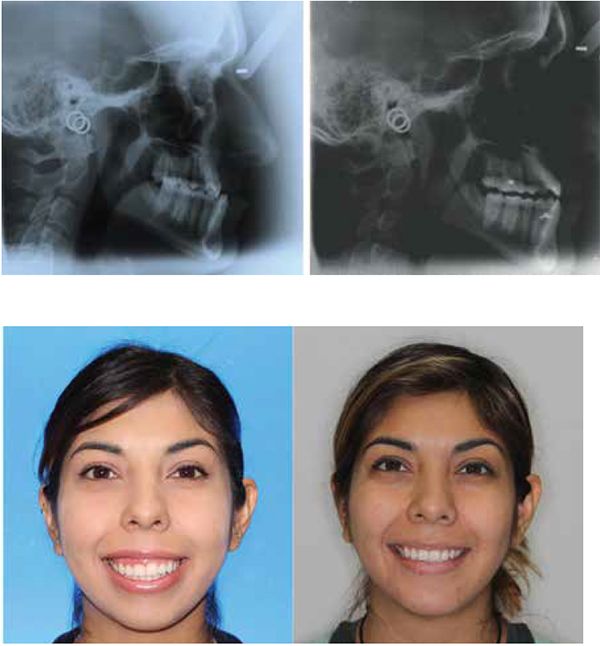
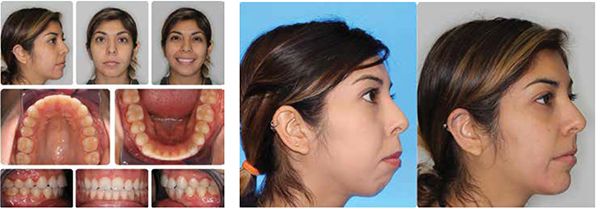
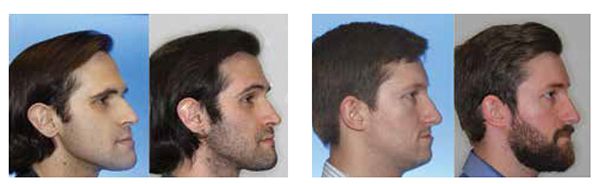
My current clinical protocol includes MOPs or vibration in conjunction with Invisalign, Incognito, Insignia/Damon and traditional metal mini twins. For patients who chose both MOPs and vibration, I am finding a 50% reduction in treatment time. With the combination of two bone modulators only a 10-15% improvement, we are finding that MOPs and/or vibration can be used on all types of difficult malocclusions, such as severe crowding or spacing (Figures 7-8), for all of our TAD cases, open bite cases (Figure 9), surgical treatment plan (Figures 11-14), and nonsurgical (Figures 9-10, 15, 16), impacted teeth,19 intrusion of gummy smiles19, extraction cases, or molar protraction (Figures 2-3).21
Practice management considerations
My initial application of MOPs was for difficult or stubborn tooth (e.g., maxillary laterals) with aligners. Treatment acceptance with MOPs is higher with existing patients who need to get completed just based on cost. All extended treatments are recommended AO as we begin to see they will not meet their estimated treatment time. Patients toward the end of treatment wanting to finish as soon as possible, find AcceleDent to be cost prohibitive and are more receptive to MOPs. Fee consideration plays a role in the doctors and patients decision. I charge $250 for each MOP treatment and $800-$1,200 for a low-frequency vibration device. I have also chosen a no-fee MOP treatment, as there is a benefit to completing treatment early (extended treatment costs are greater than the cost of the Propel tip). Patients are able to reliably accelerate their treatment with a cost-effective, minimally invasive procedure like MOPs21 or with low-frequency vibration. Some AO alternatives like Wilkodontics™ (Figure 17) or Piezosurgery (Figure 18) are not cost-effective, and require surgery at a price range of $5,000 to $10,000. There is a potential for negative sequela and extended recovery time with these surgical procedures. AcceleDent may be more costly, but vibration requires no doctor chair time, and is less clinical work. If the patient is not compliant, vibration has little to no clinical effect.

VPro5 is Propel’s new vibration device (frequency 120Hz), that is recommended for aligner seating only. The device is priced lower than AcceleDent and requires only 5 minutes per day. The idea is improved aligner seating or reduced aligner lag thus increased predictability. They do not advocate reduction in treatment time through increased rate of tooth movement, but only an improved aligner fit reducing the need for case refinement.
Biolux offers a mouthpiece with a light-activated photobiomodulation 800 nm-1000 nm wavelength, which creates enhanced tissue metabolism increasing ATP on a cellular level. Research shows 30% reduction in treatment time with photobiomodulation with Biolux.
Orthodontic fee agreements are either paid in full or spread over time. The majority of orthodontists require the full fee due by treatment finish. Patients appreciate the convenience of paying the treatment over time, as it lowers their monthly fee. Since AO has changed the original estimated treatment time, we have had to update existing contracts. This poses a new challenge with our new starts, since we don’t have as much time to amortize their fee. For some patients, we have extended the payment arrangements past the finish date, but most patients we ask to complete payment by the end of their treatment. We have added this language to our contract to assist us with this issue: “If the active phase of treatment is completed before the agreed estimated time, the full fee is due and payable at that time.” The commitment needs to be agreed upon before AO treatment is initiated.
Conclusion
In conclusion, my participation with AO has opened my eyes to the possibilities of what osseous modulation can offer orthodontic patients for the future. My traditional routines are now changing and so is my treatment planning. I am able to offer alternative treatment plans that would never be an option with traditional mechanics alone. I feel an ethical and moral obligation to inform my profession and patients of the latest technology, especially if it will make their treatment experience shorter and more pleasant. Since AO is now part of my everyday practice, I have had an increase in my capacity, due to faster finishes and a reduction in negative sequela. The benefits of decreased treatment time far outweigh any of the costs or additional work required by the patient or the practitioner utilizing AO. Performing MOPs has clinically shown to increase the rate of tooth movement or decrease the overall treatment time by 35% to 40% but has no analgesic effect and requires no compliance. Low frequency vibration can reduce treatment time by as much as 35% to 40% and offers an analgesic effect but requires compliance to be effective. Completing treatment early, with less visits, a more predictable result, and reduced negative sequela are compelling reasons to introduce AO into daily orthodontic practice.

References
- Teixeira CC, Khoo E, Tran J, et al. Cytokine expression and accelerated tooth movement. J Dent Res. 2010; 89(10):1135-1141.
- Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983(1); 31:3-9.
- Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989; 248(11):283-293.
- Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res. 1989; 248(11):294-309.
- Shih MS, Norrdin RW. Regional acceleration of remodeling during healing of bone defects in beagles of various ages. Bone. 1985; 6(5):377-379.
- Alikhani M, Raptis M, Zoldan B, et al. Effect of the micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop. 2013;144(5):639-648.
- Arias OR, Marquez-Orozco MC. Aspirin, acetaminophen, and ibuprofen: their effects on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;130(3):364-370.
- Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65(1):79-83.
- Adachi Y, Okazaki M, Ohno N, Yadomae T. Enhancement of cytokine production by macrophages stimulated with (1–>3)-beta-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol Pharm Bull. 1994; 17(12):1554-1560.
- Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic movement induces high numbers of cells expressing IFN-gamma at mRNA and protein levels. J Interferon Cytokine Res.2000; 20(1):7-12.
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223(1):20-38.
- Başaran G, Ozer T, Kaya FA, Hamamci O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2006;130(1):E1-E6.
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935-945.
- Davidovitch Z, Nicolay OF, Ngan PW, Shanfeld JL (1988). Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32(3):411-435.
- Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130(1):27-33.
- Henneman S, Von den Hoff JW, Maltha JC. Mechanobiology of tooth movement. Eur J Orthod. 2008;30(3):299-306.
- Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res. 2009;88:597-608.
- Nicozisis J. Accelerated orthodontics through micro-osteoperforation. Orthodontic Practice US. 2013;4(3):56-57.
- Guinn K. Propel orthodontics enabling faster and more predictable results. Orthotown. December 2013: 38-41.
- Pobanz JM, Storino D, Nicozisis J. Orthodontic acceleration: Propel alveolar micro-osteoperforation. Orthotown. May 2013:22-25.
- Nicozisis J. (2013) Accelerated tooth movement technology. Orthotown. July/August 2013:46-48.
- Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 2007;115(5):355-362.
- Uribe F, Padala S, Allareddy V, Nanda R. Patients’, parents’, and orthodontists’ perceptions of the need for and costs of additional procedures to reduce treatment time. Am J Orthod Dentofacial Orthop. 2014;145(4)(suppl):65-73.
- Pavlin D, Anthony R, Raj V, Gakunga P. Cyclic loading (vibration) accelerates tooth movement in orthodontic patients: a double-blind, randomized controlled trial. Seminars in Orthodontics. 2015; 21 (3):187-194.
- Bowman SJ. The effect of vibration on the rate of leveling and alignment. J Clin Orthod. 2014;48(11): 678-688.
- Ortan-Gibbs S, Kim NY. Clinical Experience with the use of pulsatile forces to accelerate treatment. J Clin Orthod. 2015; 49(9): 557-573.
- Lobre WD, Callegari BJ, Gardner G, Marsh CM, Bush AC, Dunn WJ. Pain control in orthodontics using a micropulse vibration device: a randomized clinical trial. Angle Orthod. 2016; 86(4): 625-663.
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores

 David Alpan, DDS, MSD, received his Doctor of Dental Surgery (DDS) degree from Arthur Dugoni School of Dentistry (UOP) and was licensed in California and Nevada in 1996. He earned an Orthodontic Specialty Certificate in 1998 and was awarded a Masters in Science in Dentistry (MSD) for his research on a TMJ project. Dr. Alpan founded his private practices, Alpan Orthodontics, in Los Angeles, Beverly Hills, and Las Vegas in 1999. In 2015, the Las Vegas practice was sold, and in 2016, the Century City location was added. He played an integral role for Align Technologies’ Clinical Education Department from 2002-2008 and participated as a consultant and a speaker for 6 years. He is a member of Ormco Insiders Group, Incognito Circle of Excellence, and a part of 3M Unitek’s research panel. Dr. Alpan is a Key Opinion Leader (KOL) for Propel and speaks for AcceleDent. 3D Digital Accelerated Orthodontics is his passion, so he has incorporated Propel and AcceleDent with his Insignia, Invisalign®, and Incognito daily practice. He is an active member of ADA, CDA, LADS, PCSO, AAO, CAO, AO, OKU, and TKO. His hobbies are racing cars as a member of NASA, POC, CSM, and BMW CCA, and he spends his free time with his wife, Mary, son Zephyr, and daughter Ambryn.
David Alpan, DDS, MSD, received his Doctor of Dental Surgery (DDS) degree from Arthur Dugoni School of Dentistry (UOP) and was licensed in California and Nevada in 1996. He earned an Orthodontic Specialty Certificate in 1998 and was awarded a Masters in Science in Dentistry (MSD) for his research on a TMJ project. Dr. Alpan founded his private practices, Alpan Orthodontics, in Los Angeles, Beverly Hills, and Las Vegas in 1999. In 2015, the Las Vegas practice was sold, and in 2016, the Century City location was added. He played an integral role for Align Technologies’ Clinical Education Department from 2002-2008 and participated as a consultant and a speaker for 6 years. He is a member of Ormco Insiders Group, Incognito Circle of Excellence, and a part of 3M Unitek’s research panel. Dr. Alpan is a Key Opinion Leader (KOL) for Propel and speaks for AcceleDent. 3D Digital Accelerated Orthodontics is his passion, so he has incorporated Propel and AcceleDent with his Insignia, Invisalign®, and Incognito daily practice. He is an active member of ADA, CDA, LADS, PCSO, AAO, CAO, AO, OKU, and TKO. His hobbies are racing cars as a member of NASA, POC, CSM, and BMW CCA, and he spends his free time with his wife, Mary, son Zephyr, and daughter Ambryn. 
