CEU (Continuing Education Unit): 2 Credits
Educational aims and objectives
This self-instructional course for dentists aims to provide an overview of pediatric OSA, causes, symptoms, and treatments.
Expected outcomes
Orthodontic Practice US subscribers can answer the CE questions by taking the quiz online to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Realize the prevalence of pediatric OSA and associated pathologies that can cause pediatric OSA.
- Identify some methods of screening for pediatric OSA.
- Realize the team of medical professionals that are needed for diagnosis of pediatric OSA.
- Identify possible treatments for pediatric OSA

Dr. Steven Olmos offers insights into how to approach a challenging sleep disorder in children
The prevalence of pediatric obstructive sleep apnea (POSA) has increased from 3.3%-9.4% in studies published before 2014 to 12.8%-20.4% in studies published from 2016-2023. Habitual snoring prevalence ranges from 5.3%-34.5%.1 In comparison, adults diagnosed with OSA have a worldwide prevalence of 54%.2 The difference is likely due to lack of screening and availability of pediatric sleep physicians to make a diagnosis; however, the increase in numbers demonstrates that there is a greater awareness for these conditions.
There are similar associations of pathology in children and adults such as: cardiovascular and metabolic (Type 2 diabetes) disease, and obesity. The psychosocial pathology associated with OSA is less discussed. Untreated OSA impacts affective disorders such as depression and anxiety, and often leads to decline of cognitive functions or permanent brain damage.3 Adults with OSA in the U.S. have a relatively high prevalence of depressive symptoms, and the severity of OSA positively correlates with depressive symptoms.4 In children, sleep problems predict and are predicted by generalized anxiety/depression and oppositional defiant disorder.5 There is an increased risk for depression and withdrawn/depressed symptoms in children with OSA. Arterial oxygen desaturation nadir during sleep was strongly associated with depressive symptoms over time.6
Children treated with selective serotonin reuptake inhibitors (SSRIs) can result in delayed puberty.7 Attention-deficit/hyperactivity disorder (ADHD) is among the neurobehavioral sequelae associated with OSA. A study review found that children with OSA had a high rate of attentional deficits (95%), and up to 20%-30% of children with ADHD had OSA.8 ADHD and Autism Spectrum Disorder (ASD) in children are highly comorbid with prevalence rates of ADHD in ASD being 39.4% (ages 6-11) and 38% (ages 12-17).9 Both conditions are highly comorbid with sleep disorders and improve with treatment for sleep pathology.10 The American Academy of Pediatrics recently published guidelines for screening the increased risk of adolescent suicide. They did not include screening for sleep breathing disorders. Suicide is the second leading cause of death for 10-to-24 year olds in the U.S. Individuals with neurodevelopmental disorders, including attention-deficit/hyperactivity disorder, learning disabilities, and autism spectrum disorder, are at higher risk for suicide attempts.11 Almost one-third of children and adolescents with ASD who report suicidal thoughts are aged 8 years and younger.52
Screening
In screening for POSA, it is important to look at co-morbidities. Fatigue, anxiety, and depression are most related to excessive sleepiness secondary to OSA.12
A scoping review of the last 20 years on sleep bruxism in children predicts a possible prevalence of 15%-53%.13 Sleep bruxism (SB) occurring during non-rapid eye movement (NREM) sleep and increase in microarousals is the same for adults and children in mechanism. SB activity is secondary to microarousals, with higher activity in children than adults.14 SB has a common association with OSA.15 The American Academy of Sleep Medicine classifies sleep bruxism as a movement disorder in the same category as periodic limb movement (PLM) and restless leg syndrome (RLS). It is well known that children with ADHD have elevated PLM and increased risk of OSA.16 The prevalence of elevated PLM in children with ADHD varies with a range of 26%-64%.17-19 SB, PLM, and increased EEG arousals commonly concur during sleep in a time-linked manner.20 Children with elevated PLM have more awakenings and a decrease in rapid eye movement (REM) sleep.21,22
The validated Pediatric Sleep Questionnaire (PSQ) is the most utilized screening tool. It emphasizes snoring intensity and frequency, witnessed apnea, mouth breathing, daytime fatigue, bed wetting, morning headaches, low growth, and attention deficit symptoms (Figure 1).

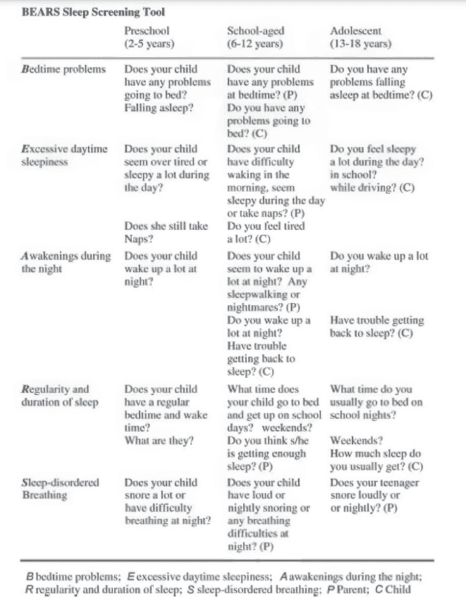
The BEARS Sleep Screening Tool developed by Judith Owens, MD (Director of Sleep Medicine, Center for Pediatric Sleep Disorders, Department of Neurology, Children’s Hospital Boston, Professor of Neurology Harvard University), divides symptoms of bedtime problems, excessive daytime sleepiness, awakenings during the night, regularity and duration of sleep, and sleep-disordered breathing by three age groups. The three age groups are around 2-5 years, 6-12 years, and 13-18 years (Figure 2).
A visual screening tool validation study has recently been concluded and submitted to the Journal of Evidence-Based Dental Practice. This study is the collaboration of this author and the principal investigator Judith Owens, MD, funded by a grant from the American Academy of Craniofacial Pain (AACP). This study looked at the risk factors for pediatric sleep-disordered breathing (SDB) and craniofacial features, validated using the PSQ and a 22-item parent reported measure for SDB risk in children. Subject characteristics included age, sex, race, and ethnicity. Various craniofacial features were evaluated, such as: retrognathic mandible, open mouth posture, convex profile, midface deficiency, flat cheeks, concave profile, dolichocephalic face, frontal asymmetry, forward head posture, and rolled shoulders. It also included intraoral evaluation of: crowding of teeth, cross-bite, narrow vaulted palate, crowding of upper teeth, narrow palate, open bite, tongue thrust, crowding of lower teeth, narrow lower jaw, tongue tie, and “heart”-shaped tip of tongue. Twelve data collection sites across the U.S. were sourced to demonstrate ease of use, reproducibility, and which conditions had highest correlation. It was found that the following conditions had the highest correlation in decreasing order: forward head posture, narrow vaulted palate, open bite, tongue thrust, tongue tie, and heart-shaped tongue.
Diagnosis
Only a physician can diagnose SDB for adults or children. The quantity and quality of diagnostic information needed to make a diagnosis is determined by the sleep specialist physician. The gold standard for the diagnosis of POSA is polysomnography (PSG); however, certain home sleep study (HST) units have been FDA-cleared. Interestingly, sleep specialty training covers both adults and children. The graduates decide their focus with the greater number treating adults, making the access to a pediatric diagnosis limited. The HST units that utilize chest and abdomen elevation belts, nasal airflow, temperature (thermistors), pulse oximetry, body position, and snoring are closest to the data collected by PSG. Many of the digital evaluation systems are based on algorithms a distance from PSG data collection. They may be used as screening tools, and their validation will be confirmed in future studies. Most diagnoses are focused on AHI>1 for POSA; however, hypercapnia is of equal concern in pediatric patients with SDB. Hypercapnia is an elevation of blood carbon dioxide secondary to abnormalities in the heart or lungs, such as respiratory acidosis, altered acid-base balance, or inadequate ventilation of the alveoli. This demonstrates why flow limitation (respiratory distress) is important in the data collection. The diagnosis of hypercapnia is made when the end tidal carbon dioxide (ETCO2) exceeds 45 mmHg during sleep.23 Children are more likely than adults to suffer from hypercapnia when they sleep.24
Treatment
Deciding upon treatment goals will help determine a comprehensive and long-term treatment plan. The late seminal figure in pediatric sleep medicine, Dr. Christian Guilleminault, wrote that the ultimate treatment goal in POSA is “restoration of nasal breathing during wake and sleep.”25
The first line of treatment for POSA is usually tonsil/adenoid (T&A) surgery; however, numerous studies have demonstrated that it may not be as successful as once thought.26-32
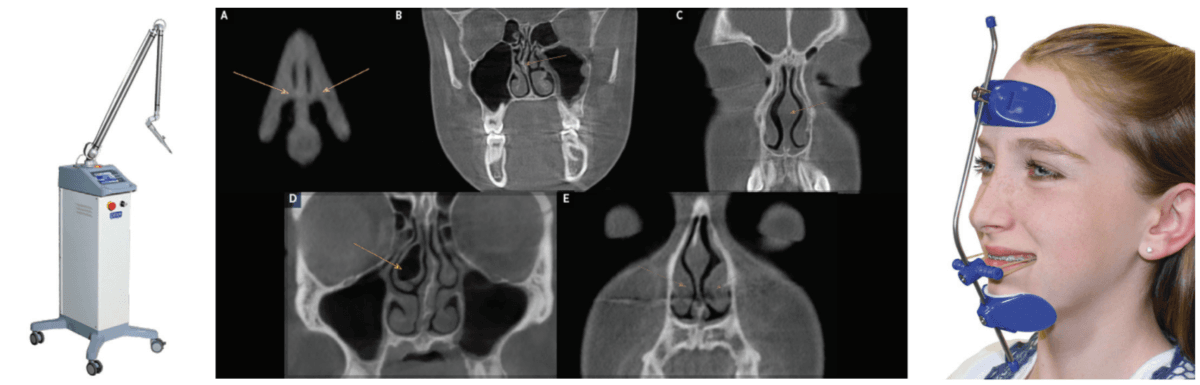
The only study to follow children post-T&A surgery over a 3-year period (with systematic evaluation using PSG at 6, 12, 24, and 36 months) showed that there was a 75% relapse of SD after 6 months.33 A pilot study utilizing CO2 laser (Deka/Bio Research Inc., Figure 3) to shrink palatal and base of tongue tissue for incomplete responders of oral appliance therapy (OAT) for OSA is currently underway at the University of Alberta School of Dentistry, Edmonton, Canada, by the principal investigator Enoch T. Ng, DDS, PhD (Figure 3). He has published a case report on the effectiveness of photobiomodulation for pediatric hypertrophic tonsils.34 These devices may someday be FDA-cleared to shrink tonsils. The morbidity and mortality of T&A surgery could be replaced with dentists performing a simple and painless technique.
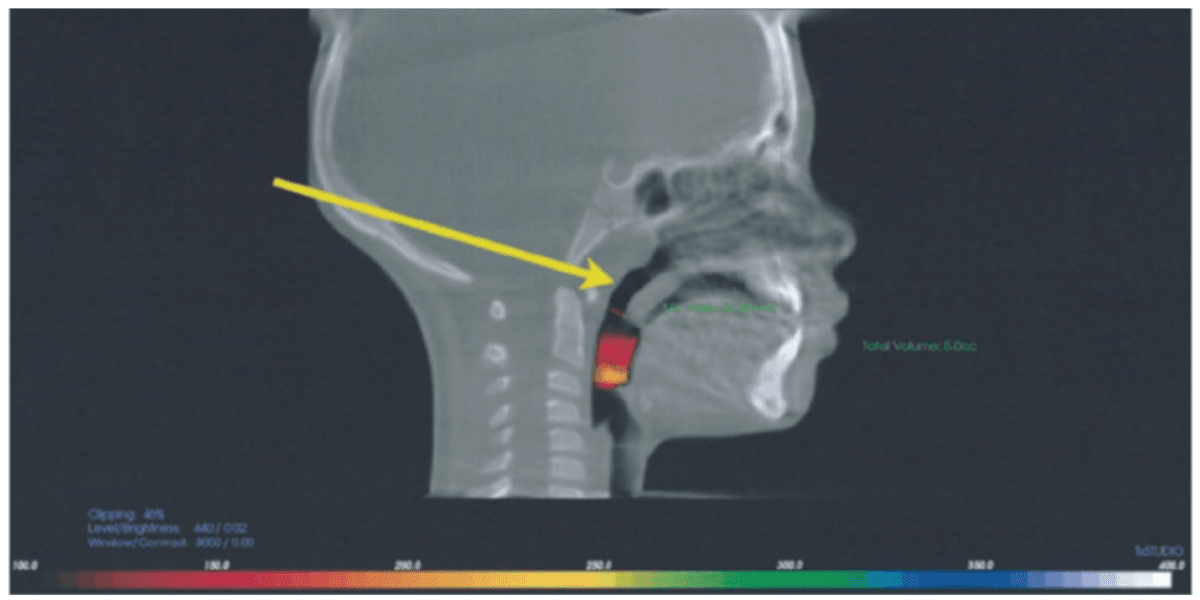
A second line treatment to treat POSA is positive airway pressure therapy (PAP), most often utilizing continuous positive airway pressure (CPAP). Midface deficiency and POSA are highly comorbid. This is demonstrated by the incidence of children with Down syndrome who have POSA after T&A surgery at 65%-73%.35-38 CPAP use on children with POSA has been shown to prevent normal maxillary development and increase midface deficiency, thus making the patient more dependent on PAP.39 Maxillary development has been demonstrated to improve POSA and nasal function by increasing volume.40,41 It has been shown that maxillary expansion can shrink T&A and increase the internal nasal valve (which is the first point of entry of air into the nose).42,43 Nasal valve compromise (narrowing of the internal nasal valve) has been found to result in a 7-times greater chance of TM joint capsulitis and facial and cervical myositis, via mouth breathing, when compared to other nasal obstructions (Figure 4).44
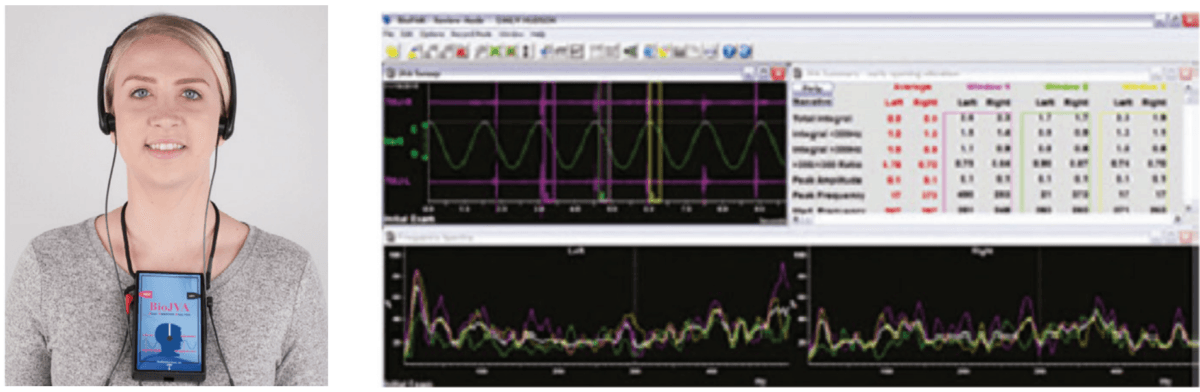
Protraction of the maxilla in combination with transverse development utilizing a Delaire’s mask for Class III patients with POSA has been shown to produce statistically significant increase in linear upper airway measurements and oropharyngeal and nasopharyngeal dimensions, improving airway patency and OSA (Figure 5).45 The problem with this technique is that it uses the frontal bone and the mandible as leverage, placing retractive forces on them. If the patient has articular disc displacement and or capsulitis (inflammation of the joint capsule), the retraction of the mandible can result in increased pathology. A proper screening of the TM joints via joint vibration analysis to measure soft and hard tissue vibrations during function (JVA BioResearch, Inc.), as well as a thorough clinical examination, will help to identify these patients (Figures 6 and 7).
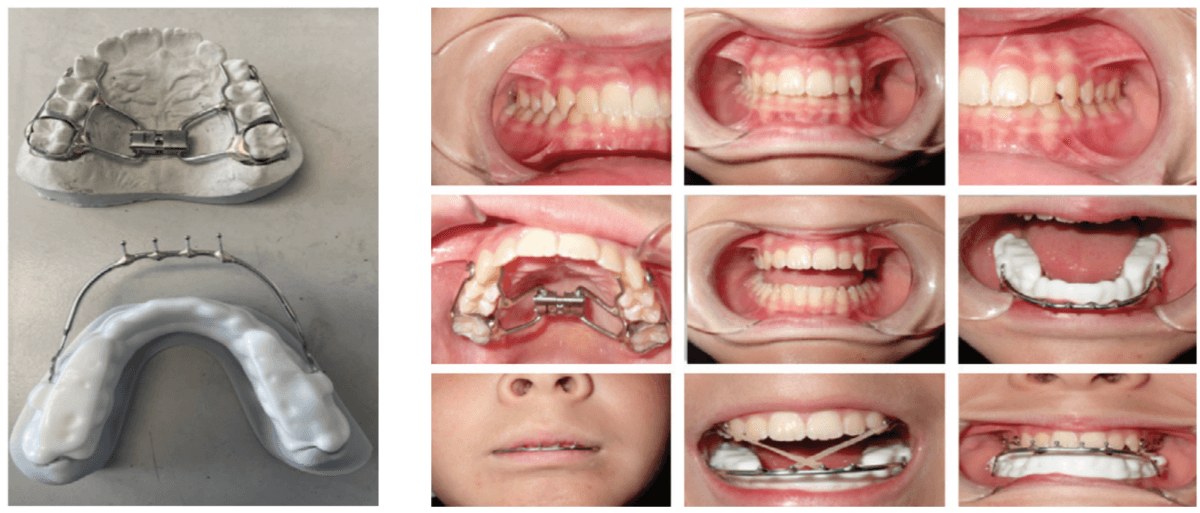
Decompressing the TM joints with a removable lower appliance at a physiologic position (sibilant phoneme registration/phonetic bite) has been shown to increase pharyngeal airway volume and decrease collapse. It also allows for stabilization of the TM joints, reducing pressure on the mandible without any pressure on the frontal bone.46,47 This Tandem Bow technique was explained in a previous case study titled, “Pediatric severe apnea/obesity/TMD/headache-Class III” in Orthodontic Practice US, May/June 2016, Volume 7, No. 3, pages 20-24. The 10-year-old patient who had been on CPAP for 5 years with an AHI of 118 was reduced to an AHI of 3 in 8 weeks and was able to discontinue her PAP therapy (Figure 8). Forward head posture corrections can be seen with decompression of the TM joints utilizing this technique (Figure 9). This technique is explained in Chapter 7 of Sleep Disorders in Pediatric Dentistry: Clinical Guide on Diagnosis and Management, edited by Dr. Edmund Liem, and published by Springer. Chapter 7 is authored by Dr. Edmund A. Lipskis, and titled, “Orthodontic and Dentofacial Orthopedic Treatment Strategies for Pediatric Sleep Disorders.” Advancing the entire maxilla is necessary to protract the palatine bones to increase the volume of the velopharynx (the narrowest part of the airway) (Figure 10).
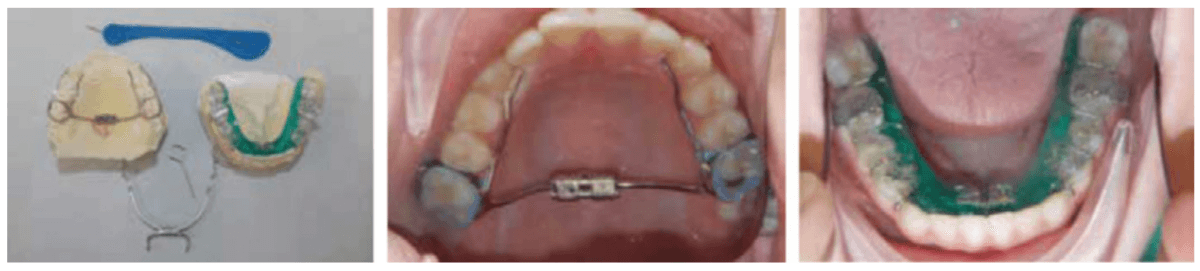

The lower appliance that receives the Tandem Bow is to be worn 12 hours per day and only removed to eat if necessary. Most children should sleep for 10 hours, so the device can be worn during sleep, an hour before bed and an hour after waking. The force of the elastics, worn for 12 hours per day (mostly while sleeping), should have approximately 200g per side in mixed dentition and 250-300g per side in permanent dentition (Figure 11).
When used on mixed dentition, there will be a need to place extra buccal retention on the lower deciduous molars for the “C” clasps (Figures 12 and 13).
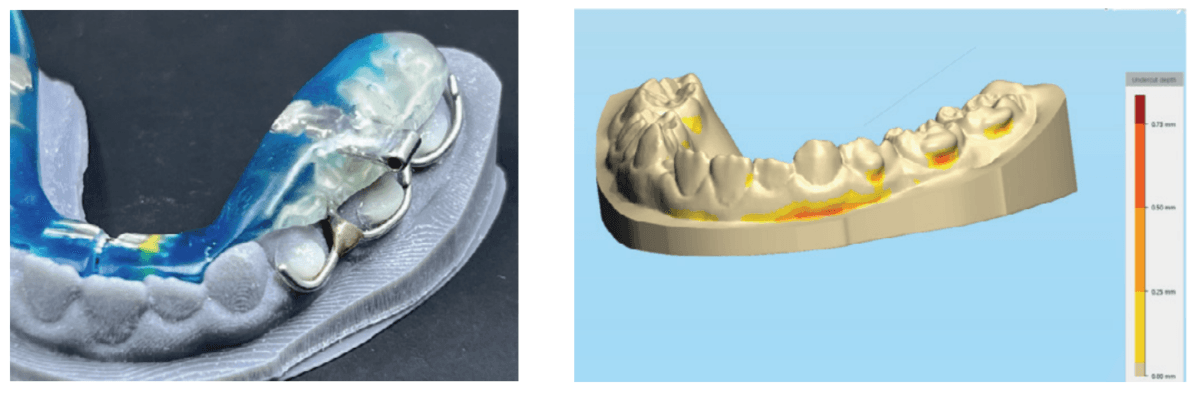
The evolution of printed biofriendly materials produced by Diamond Orthotic Lab for removable orthopedic development and facial pain has greatly improved treatment (Figures 14 and 15). These appliances are made of Type 12 nylon and are thermally active, making them easy to adjust with either hot or cold water.
Myofunctional therapy for POSA has been shown to reduce AHI by 62%.48 This type of therapy is ideal primary treatment for 2- to 5-year-old patients exclusively. It is also utilized as a retentive therapy for post-orthopedic development. Myofunctional Research Co. (MRC) has a range of products, including oral appliances (Myobrace®), lip and tongue strength exercise devices (myotalea®), and pharyngeal airway development (Myosa®) (Figures 16, 17, and 18).
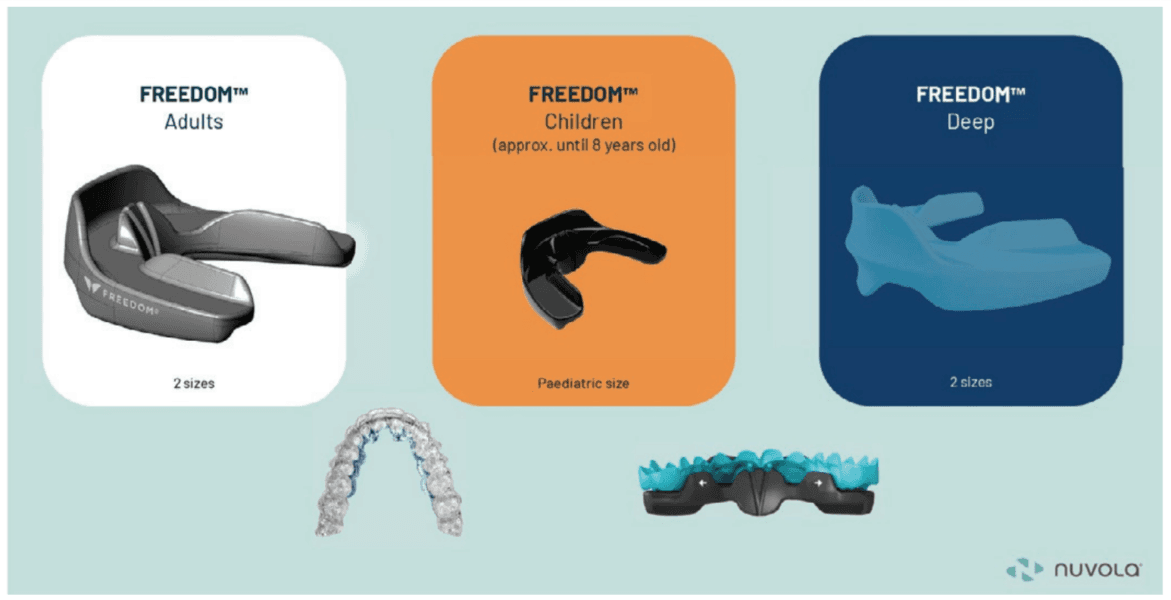
A new technique to North America, incorporating orthopedic development of the maxilla in combination with aligner therapy, is called Nuvola (BioMax). It was developed in Italy by Dr. Alessandro Carrafiello, an orthodontist/physician with remarkable treatment outcomes for both adults and children. Children as young as 5 can benefit from this treatment. This treatment utilizes a chewable oral device (Freedom appliance) that is used for only 30 minutes per day. The Freedom device comes in three types: regular, deep bite, and pediatric (Nuvola Jr). This device uses piezoelectric forces to develop the maxilla and mandible in combination with reinforced aligners, without the need for fixed appliances (Figure 19). It is tremendously easy to implement for practitioners and patients. Compliance is much higher than in previous treatments for malocclusion. There are currently no studies on how effective this treatment is for POSA; however, it looks promising.
Photobiomodulation (laser) treatment has been shown to accelerate orthodontic movement and reduce inflammation and pain from orthodontic therapy.49-51 Studies are being developed to combine laser therapy using NdYag (Deka/BioResearch Inc.) and MLS (BioResearch Inc.) and these outlined treatments for orthopedic and orthodontic movement, allowing shorter treatment times and maximum treatment outcomes (Figures 20 and 21). The future is now.
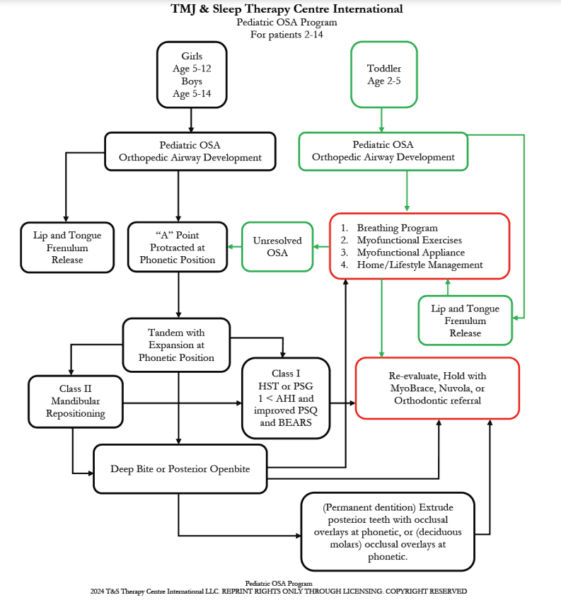
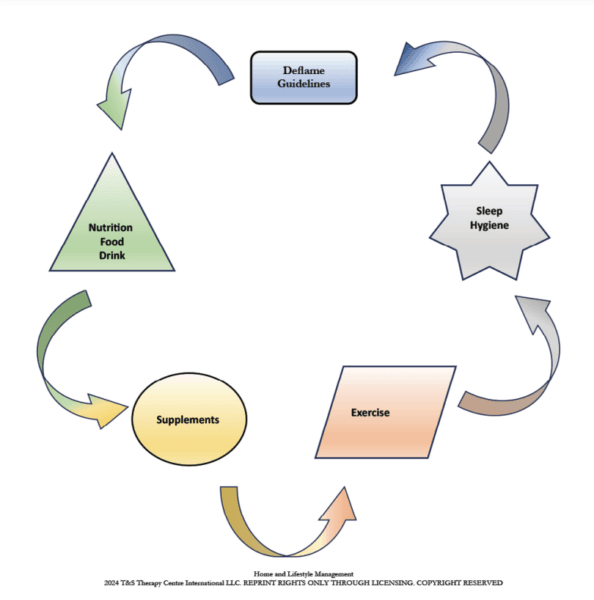
A proposed treatment outline for POSA might follow the timeline in Figure 22. A comprehensive treatment approach also includes home and lifestyle management (Figure 23).
Treating POSA has far greater effects than simply restoring restful sleep. The arguably more important effect may be in saving a child’s life. The psychosocial consequences illustrated in this article are rarely mentioned in the treatment of POSA and should motivate us to screen and treat patients as early as possible.
For training courses in the various methods outlined in this article, please visit our website www.tmjtherapycentre.com.
Whether you offer treatment for pediatric sleep disorders or for adults, it is always helpful to read “Basic principle review of sleep medicine,” by Drs. Barry Glassman and Don Malizia. https://orthopracticeus.com/ce-articles/basic-principle-review-of-sleep-medicine/.
References
- Magnusdottir S, Hill EA. Prevalence of obstructive sleep apnea (OSA) among preschool aged children in the general population: A systematic review. Sleep Med Rev. 2024 Feb;73:101871.
- de Araujo Dantas AB, Gonçalves FM, Martins AA, Alves GÂ, Stechman-Neto J, Corrêa CC, Santos RS, Nascimento WV, de Araujo CM, Taveira KVM. Worldwide prevalence and associated risk factors of obstructive sleep apnea: a meta-analysis and meta-regression. Sleep Breath. 2023 Dec;27(6):2083-2109.
- Vanek J, Prasko J, Genzor S, Ociskova M, Kantor K, Holubova M, Slepecky M, Nesnidal V, Kolek A, Sova M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020 Aug;72:50-58.
- Li M, Zou X, Lu H, Xin Y, et al. Association of sleep apnea and depressive symptoms among US adults: a cross-sectional study. BMC Public Health. 2023;23:427.
- Shanahan L, Copeland WE, Angold A, Bondy CL, Costello EJ. Sleep problems predict and are predicted by generalized anxiety/depression and oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2014 May;53(5):550-558.
- Hodges E, Marcus CL, Kim JY, Xanthopoulos M, Shults J, Giordani B, Beebe DW, Rosen CL, Chervin RD, Mitchell RB, Katz ES, Gozal D, Redline S, Elden L, Arens R, Moore R, Taylor HG, Radcliffe J, Thomas NH. Depressive symptomatology in school-aged children with obstructive sleep apnea syndrome: incidence, demographic factors, and changes following a randomized controlled trial of adenotonsillectomy. 2018 Dec 1;41(12):zsy180.
- Alotoom OM, Marwaha R. Association Between SSRIS And Delayed Puberty In Children. Jour Amer Acad Child & Adolesc Psych. Oct 2023;62(10):S196.
- Ivanov I, Miraglia B, Prodanova D, Newcorn, JH. Sleep Disordered Breathing and Risk for ADHD: Review of Supportive Evidence and Proposed Underlying Mechanisms. Journal of Attention Disorders. 2024;28(5):686-698.
- Jones Piltz V, Halldner L, Markus J-F, Fridell A, Bolte S, Olsson NC. Symptom similarities and differences in social interaction between autistic children and adolescents with and without ADHD. Curr Psychol. 2024;43:3503-3513.
- Berenguer C, Baixauli I, Rosa E, De Stasio S. Sleep problems in children with autism spectrum disorder and attention-deficit/hyperactivity disorder: A comparative study and effects on communication skills. Autism Res. 2024 Feb;17(2):355-365.
- Hua LL, Lee J, Rahmandar MH, Sigel EJ; Committee On Adolescence; Council On Injury, Violence, And Poison Prevention. Suicide and Suicide Risk in Adolescents. 2024 Jan 1;153(1):e2023064800.
- Mjelle KES, Lehmann S, Saxvig IW, Gulati S, Bjorvatn B. Association of Excessive Sleepiness, Pathological Fatigue, Depression, and Anxiety With Different Severity Levels of Obstructive Sleep Apnea. Front Psychol. 2022 Mar 31;13:839408.
- Huynh N, Fabbro CD. Sleep bruxism in children and adolescents-A scoping review. J Oral Rehabil. 2024 Jan;51(1):103-109.
- Restrepo C, Lobbezoo F, Castrillon E, Svensson P, Santamaria A, Manfredini D. Correlations between sleep architecture and sleep-related masseter muscle activity in children with sleep bruxism. J Oral Rehabil. 2024 Jan;51(1):110-116.
- American Academy of Sleep Medicine. In: International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine;2014.
- Youssef NA, Ege M, Angly SS, Strauss JL, Marx CE. Is obstructive sleep apnea associated with ADHD? Ann Clin Psychiatry. 2011 Aug;23(3):213-224.
- Crabtree VM, Ivanenko A, O’Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003 Mar;12(1):73-81.
- Chervin RD, Archbold KH, Dillon JE, Pituch KJ, Panahi P, Dahl RE, Guilleminault C. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. 2002 Mar 15;25(2):213-218.
- Picchietti DL, England SJ, Walters AS, Willis K, et al . Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13(12):588-594.
- van der Zaag J, Naeije M, Wicks DJ, Hamburger HL, Lobbezoo F. Time-linked concurrence of sleep bruxism, periodic limb movements, and EEG arousals in sleep bruxers and healthy controls. Clin Oral Investig. 2014;18(2):507-513.
- Crabtree VM, Ivanenko A, O’Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003 Mar;12(1):73-81.
- Gingras JL, Gaultney JF, Picchietti DL. Pediatric periodic limb movement disorder: sleep symptom and polysomnographic correlates compared to obstructive sleep apnea. J Clin Sleep Med. 2011 Dec 15;7(6):603-609A.
- Almanza-Hurtado A, Polanco Guerra C, Martínez-Ávila MC, Borré-Naranjo D, Rodríguez-Yanez T, Dueñas-Castell C. Hypercapnia from Physiology to Practice. Int J Clin Pract. 2022 Sep 23;2022:2635616.
- Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. 2008 Jul;122(1):e149-155.
- Guilleminault C, Sullivan SS. Towards Restoration of Continuous Nasal Breathing as the Ultimate Treatment Goal in Pediatric Obstructive Sleep Apnea. Enliven: Ped & Neonatal Bio. Sept 2014;1(1).
- Guilleminault C, Partinen M, Praud JP, Quera-Salva MA, Powell N, Riley R. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr. 1989 Jun;114(6):997-999.
- Tasker C, Crosby JH, Stradling JR. Evidence for persistence of upper airway narrowing during sleep, 12 years after adenotonsillectomy. Arch Dis Child. 2002 Jan;86(1):34-37.
- Guilleminault C, Li K, Quo S, Inouye RN. A prospective study on the surgical outcomes of children with sleep-disordered breathing. 2004 Feb 1;27(1):95-100.
- Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O’Brien LM, Ivanenko A, Gozal D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006 Dec;149(6):803-808.
- Guilleminault C, Huang YS, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg. 2007 Feb;136(2):169-75.
- Amin R, Anthony L, Somers V, Fenchel M, McConnell K, Jefferies J, Willging P, Kalra M, Daniels S. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008 Mar 15;177(6):654-659.
- Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, Kaditis AG, Splaingard D, Splaingard M, Brooks LJ, Marcus CL, Sin S, Arens R, Verhulst SL, Gozal D. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010 Sep 1;182(5):676-683.
- Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. 2012 Apr;129(4): e857-865.
- Ng ET, Lagravere MO, David A. Photobiomodulation for pediatric hypertrophic tonsils: a clinical case report. Pediatr Med 2021;4:40. https://dx.doi.org/10.21037/pm-21-18.
- Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: Should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006 Apr;132(4):432-436.
- Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome. 2016 Mar 1;39(3):699-704.
- Hill CM, Evans HJ, Elphick H, Farquhar M, Pickering RM, Kingshott R, Martin J, Reynolds J, Joyce A, Rush C, Gavlak JC, Gringras P. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016 Nov-Dec;27-28:99-106.
- Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome. Arch Pediatr Adolesc Med. 2003 Jul;157(7):655-660.
- Roberts SD, Kapadia H, Greenlee G, Chen ML. Midfacial and Dental Changes Associated with Nasal Positive Airway Pressure in Children with Obstructive Sleep Apnea and Craniofacial Conditions. J Clin Sleep Med. 2016 Apr 15;12(4):469-475.
- Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. 1998 Dec 15;21(8):831-835.
- Motro M, Schauseil M, Ludwig B, Zorkun B, Mainusch S, Ateş M, Küçükkeleş N, Korbmacher-Steiner H. Rapid-maxillary-expansion induced rhinological effects: a retrospective multicenter study. Eur Arch Otorhinolaryngol. 2016 Mar;273(3):679-87.
- Yoon A, Abdelwahab M, Bockow R, Vakili A, Lovell K, Chang I, Ganguly R, Liu SY, Kushida C, Hong C. Impact of rapid palatal expansion on the size of adenoids and tonsils in children. Sleep Med. 2022 Apr;92:96-102.
- Yoon A, Abdelwahab M, Liu S, Oh J, et al. Sleep and Breathing 2021;25:1019-1027.
- Olmos SR. Nasal airway obstruction and orofacial pain: a multicenter retrospective analysis. Gen Dent. 2022 Nov-Dec;70(6):28-33.
- Caruso S, Lisciotto E, Caruso S, Marino A, Fiasca F, Buttarazzi M, Sarzi Amadè D, Evangelisti M, Mattei A, Gatto R. Effects of Rapid Maxillary Expander and Delaire Mask Treatment on Airway Sagittal Dimensions in Pediatric Patients Affected by Class III Malocclusion and Obstructive Sleep Apnea Syndrome. Life (Basel). 2023 Mar 1;13(3):673.
- Singh GD, Olmos S. Use of a sibilant phoneme registration protocol to prevent upper airway collapse in patients with TMD. Sleep Breath. 2007 Dec;11(4):209-216.
- Olmos SR, Kritz-Silverstein D, Halligan W, Silverstein ST. The effect of condyle fossa relationships on head posture. 2005 Jan;23(1):48-52.
- Camacho M, Certal V, Abdullatif J, Zaghi S, Ruoff CM, Capasso R, Kushida CA. Myofunctional Therapy to Treat Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. 2015 May 1;38(5):669-675.
- Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med. 2004;35(2):117-120.
- Doshi-Mehta G, Bhad-Patil WA. Efficacy of low-intensity laser therapy in reducing treatment time and orthodontic pain: a clinical investigation. Am J Orthod Dentofacial Orthop. 2012 Mar;141(3):289-297.
- Qamruddin I, Alam MK, Mahroof V, Fida M, Khamis MF, Husein A. Effects of low-level laser irradiation on the rate of orthodontic tooth movement and associated pain with self-ligating brackets. Am J Orthod Dentofacial Orthop. 2017 Nov;152(5):622-630.
- Schindel BJ, Baer Chen B, Wilcox HC, Marvin AR, Law JK, Lipkin PH. Suicidal thoughts and behaviors among children and adolescents with autism spectrum disorder. JAMA Pediatr. Published online April 1, 2004. doi:10.1001/jamapediatrics.2004.0207
Stay Relevant With Orthodontic Practice US
Join our email list for CE courses and webinars, articles and mores


